Chemistry Reference
In-Depth Information
oxidation with bromine water and the catalytic method using the reaction between Ce(IV)
and As(III) [82]. Variance tests showed that differences between either replicates or
methods was not significant (Fig. 3.5).
Truesdale and Smith [81] also carried out a comparative study of the determination of
iodate in open ocean, inshore Irish seawaters and waters from the Menai Straits, using the
spectrophotometric method (with and without pre-oxidation using iodine water) and also
by a polarographic method [83].
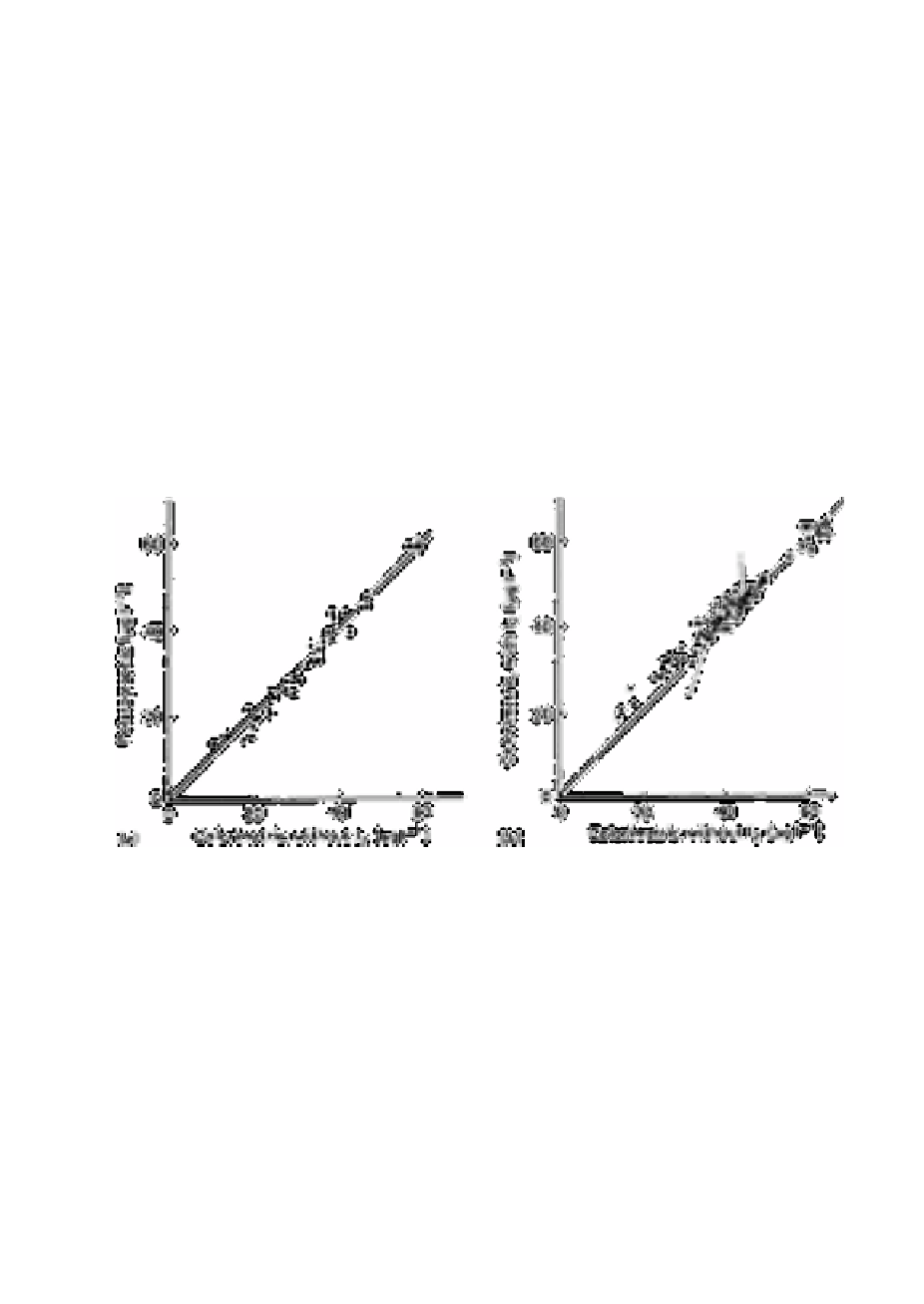
Fig. 3.6 shows the results obtained in a comparison of these methods on a range of
deep-sea and offshore samples. The line of gradient 1.0 on each diagram shows the result
which would have occurred had agreement been obtained. The Students t-test (Table 3.7)
showed that in both exercises the colorimetric method with iodine-water treatment
yielded higher values than that without iodine-water treatment, and the polarographic
method yielded, on average, a concentration lower than that obtained by the colorimetric
procedure without iodine-water.
Schnepfe [84] has described yet another procedure for the determination of iodate and
total iodine in seawater. To determine total iodine, 1ml 1% aqueous sulphamic acid is
added to 10ml seawater which, if necessary, is filtered and then adjusted to a pH of less
than 2.0. After
Fig. 3.6
The distribution of sample concentrations encountered in the
comparisons of the colorimetric method without iodine with (a) the
polarographic method and (b) the colorimetric method with iodine
water. For convenience the line of unit gradient is shown in both
cases. In each case the precision (95% confidence level) for each
measurement is as shown for the most concentrated sample. In (b) the
broken line divides onshore (∆) and offshore ( ) samples
Source: Reproduced with permission from Elsevier Science [81]
Table 3.7
Results of testing the difference in the means obtained by the different iodate
methods


Search WWH ::

Custom Search