Chemistry Reference
In-Depth Information
In all three methods a blank is obtained. To ascertain the blank excess sodium
thiosulphate is added to the potassium iodide reagent at a concentration of 4.0×10
−14
mol
L
−1.
Samples were reanalysed and the appropriate blank subtracted from the sample
signal.
Variations in the salinity of the sample have very little effect on the accuracy of the
results obtain ed in all three methods provided that the difference in salinity between the
samples and the standards does not exceed 2.5 ‰.
Determinations of iodate without pre-oxidation in Pacific seawater by the above
method gave a mean result of 583µg L
−1
with a standard deviation of 0.23µg L−1. For
samples containing between 40 and 60µg L
−1
standard deviations of 0.19µg L
−1
(iodate
method with pre-oxidation), 0.12µg L−
1
(iodate method without pre-oxidation) and
0.43µg L−
1
(total iodine method) were obtained.
A set of Pacific open-ocean samples were analysed for iodate-iodine using both the
procedure which incorporates pre-oxidation with iodine-water and that which does not.
Also, in a similar exercise total-iodine was determined using both the method that
incorporates pre-
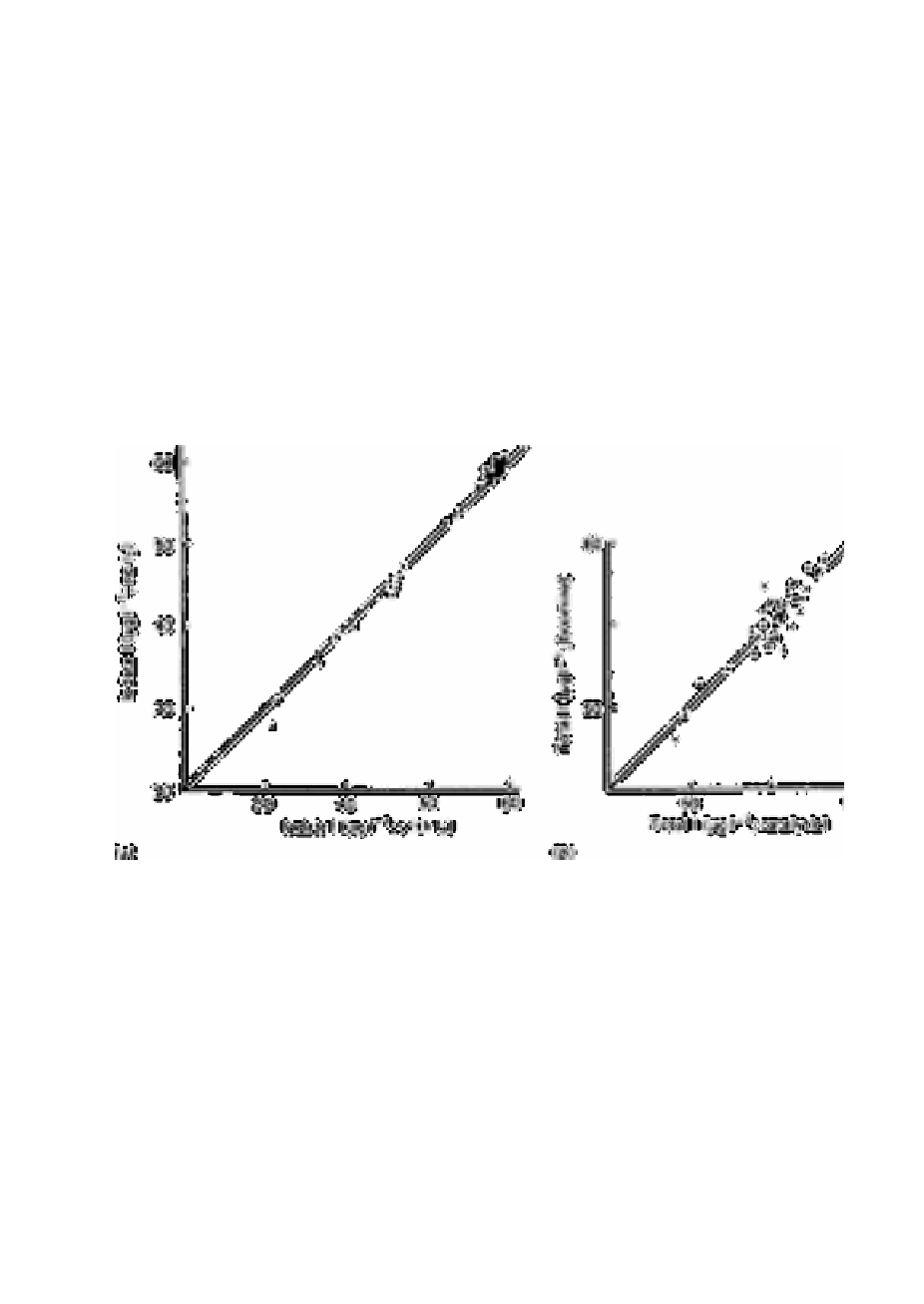
Fig. 3.5
The distribution of sample concentrations (means) encountered in the
comparison of methods exercised (a) for iodate-iodine with and
without iodine-water pre-oxidation; (b) for total iodine using the
bromination procedure and older catalytic procedure. The analysis of
variance has shown that in both exercises there was no significant
difference between methods; any apparent bias is not significant
when the variance due to sample replication is included. For
convenience the line of unit gradient is shown. Note that an expanded
scale has been used in the plot for total-iodine results
Source: Reproduced with permission from Elsevier Science [81]
Search WWH ::

Custom Search