Chemistry Reference
In-Depth Information
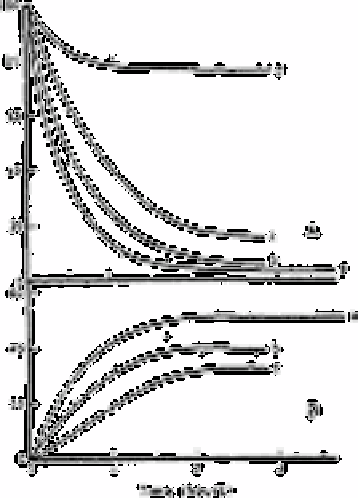
Fig. 3.3
(a) Disappearance with time of residual oxidants and (b) concomitant
appearance of bromate (equation (2) above) in chlorinated seawater
(4.2 to 4.9ppm of Cl2) as a function of exposure to sunlight. The
conditions were: Curve (a)) full midday sunlight, Curve (b) 65% of
full sunlight, and curve (c) overcast, 20% of full sunlight. Curve (d)
shows residual oxidant disappearance in the dark at 40°C. no bromate
production was observed in the dark.
Source: Reproduced with permission from Springer-Verlag [18]
conversion of chlorine). Addition of copper sulphate to give a cupric ion concentration in
the seawater of 100 parts per billion did not induce measurable bromate production in the
dark. Curve (b) was obtained from a chlorinated (4.9mg L
−1
) seawater solution that was
exposed to full sunlight for 70min. Curve (c), which is offset by 0.4µa with respect to
curves (a) and (b), shows 1.0×10
−5
M sodium bromate in seawater.
Fig. 3.3 illustrates kinetic data for the appearance of bromate (Fig 3.3(a)) and
disappearance of residual oxidants (Fig. 3.3(b)) in chlorinated seawater exposed to
sunlight. Curves (a) were obtained from solutions exposed to full midday sunlight for the
duration of the experiment: curves (b) are for exposure to partial sunlight (the average
light intensity was approximately 65% of full sunlight); and curves (c) are for overcast
conditions (average light intensity, 20% of full sunlight). Curve (d) in Fig. 3.3(a) shows
the disappearance of residual oxidants with time at 40°C in the dark. The ordinates are
calibrated as the percentage of the added chlorine recovered as residual oxidants (Fig. 3.3
(a)) or as bromate formed according to equation (2) above (Fig. 3.3(b)).
The lack of observable bromate production in the dark is not inconsistent with the
Search WWH ::

Custom Search