Chemistry Reference
In-Depth Information
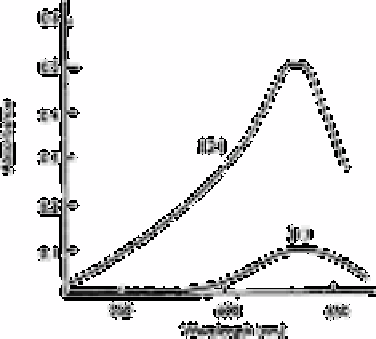
Fig. 3.1
Absorption spectra: (1) reagent blank; (2) 6.25×10
−6
mol
L−1
boric
acid. Conditions: 2.5×10
−4
mol L
−1
malachite green, 2.5×10
−2
mol
L
−1
mandelic acid, pH 3.0; benzene as reference
Source: Reproduced with permission from Elsevier Science [16]
Fig. 3.1. Malachite green itself was not extracted into benzene irrespective of the
presence or absence of boron, when mandelic acid was absent. The wavelength of
maximum absorption of each spectrum occurs at 633nm.
3.9
Bromate
3.9.1
Spectrophotometric titration and differential pulse polarography
Chlorinated waters are being discharged to estuaries and coastal waters in increasing
quantities. In such systems the chlorine reacts with the natural bromide and ammonia at
pH 8 to produce the highly toxic hypobromous acid, hypobromite ion and haloamines.
For normal seawater of pH 8, the initial products of chlorination are a mixture of
hypobromous acid and hypobromite ion. Both of these compounds are unstable with
respect to decomposition and disproportionation.
Macalady
et al.
[17] report experiments in which chlorinated sea water was exposed to
sunlight at various intensities, to simulate different periods of the day. The results of
subsequent analyses for bromates and residual oxidants show that the rate and extent of
bromate formation depend on the intensity of the sunlight, with none found in samples

Search WWH ::

Custom Search