Chemistry Reference
In-Depth Information
blank; (3) orthophosphate (P: 5ng); (4) orthophosphate (P: 10ng); (5) sample
solution (P: 8.5ng)
Source: Reproduced with permission from Springer Verlag [651]
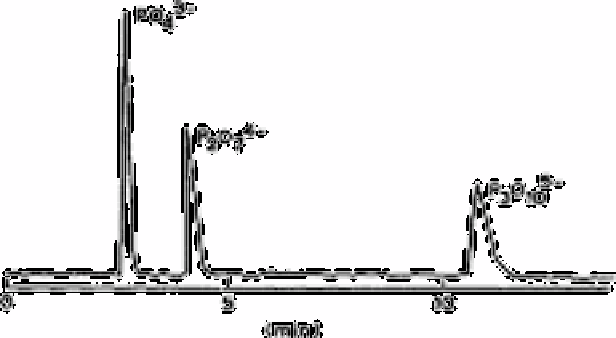
Fig. 2.42
Chromatogram of orthophosphate, diphosphate and triphosphate.
Column is µ-Gondapak-NH
2
and the eluant was oxalate-Mg buffer at
the flow rate of 1.0ml min
−1
, 50µL of mixed solution which contains
10
−3
M each phosphate
Source: Reproduced with permission from Springer Verlag [651]
plus magnesium and acetate plus magnesium gave poor resolutions. Oxalate plus
magnesium buffer should be freshly prepared as it is subject to decomposition. The
column should be rinsed after use.
In Fig. 2.42 is shown a typical chromatogram, obtained by this technique for a mixture
of orthophosphate, pyrophosphate and P
3
O
10
5−
. Morgan and Danielson [652] have
described the development of an enzymatic method of determining phosphate eluted from
a reverse phase high performance liquid chromatographic column. The method is
applicable to sewage effluents and non saline water samples. It is based on the nucleoside
phosphorylase catalysed conversion of inosine and orthophosphate to hypoanthine. This
method is claimed to give better results than standard molybdenum blue
spectrophotometric methods.
The inosine and hypoxanthine were separated by reversed phase high performance liquid
chromatography and the amount of hypoxanthine produced was related to the phosphate
concentration. Quantitation of the hypoxanthine peak was found to be linear with
orthophosphate up to about 30mg L
−1
. A detection limit of 0.75mg L
−1
could be obtained
after dialysis of the commercial enzyme. Interference studies showed that the enzymatic
assay unlike the colorimetric molybdate blue technique was essentially unaffected by

Search WWH ::

Custom Search