Chemistry Reference
In-Depth Information
The technique used malachite green in acidic medium as chromogenic reagent and
spectrophotometric measurements are made at 650nm (Fig. 2.38). The procedure is very
sensitive and can be used at a high sampling rate.
In Table 2.44 tolerable levels of coexisting ions likely to occur in river water samples
are shown.
Only orthophosphate can be determined by this method. However, the condensed
phosphates can easily be hydrolysed to orthophosphate by
Reagent solution. Dissolve 19.4g of ammonium heptamolybdate tetrahydrate,
0.092g of malachite green (oxalate) in about 500ml of distilled water, add 250ml
of ethanol and 70ml of concentrated sulphuric acid and dilute to 1000ml with
distilled water. This solution is 0.1 1mol L
−1
in molybdenum and 2.2×10
−4
mol
L
−1
in malachite green. Filter through a 0.45µm membrane filter before use.
This solution is stable for at least one month if stored in the dark.
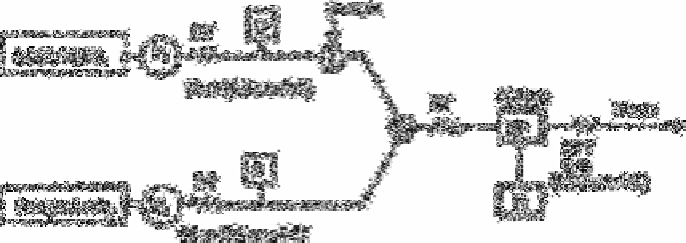
Fig. 2.38
Schematic flow diagram. P1 and P2, pumps; DT, damping coil
(0.5mm×10m); D, air damper; 1, sample injector; M, mixing joint;
RT, reaction coil (1mm×7.5m or 0.5mm× 5m); SP,
spectrophotometer with flow cell; R, recorder; BPT, back pressure
coil (0.25cm ×3m)
Source: Reproduced with permission from Elsevier Science [632]
Table 2.44
Tolerable concentrations of coexisting ions. Phosphorus taken: 120µg L
−1
Maximum tolerable concentration,
mol L
−1
Ion
Na
+
, Cl
−
0.1
Mg
2+
, Ca
2+
0.01
Al
3+
, K
+
, Fe
3+
, NO
3
−
, HCO
3
=10
−3a
SiO
3
2-
10
−4
WO
4
2−
,VO
3
-
10
−5
AsO
4
3−
2×10
−6
a
Maximum tested



Search WWH ::

Custom Search