Chemistry Reference
In-Depth Information
The NADPH
2
formed is determined by measuring its fluorescence. This method is very
sensitive because in terms of amount there is no real sensitivity limit as the NADPH
2
formed can be measured by enzymic cycling. In terms of concentration, the sensitivity is
limited mainly by the degree to which phosphate can be removed from the reagents. As
the analytical reaction takes place under neutral conditions, inorganic phosphate can be
measured in the presence of very unstable organic phosphate.
Land and Edmonds [625] have studied fluorescence quenching by phosphate on
aluminium morin, aluminium-3-hydroxyflavone, gallium-morin, gallium-3-
hydroxyflavone, gallium-quercetin, zirconium-morin and zirconium-3-hydroxyflavone
systems. They have found that only the first of these is suitable for determining the
phosphate ion. The percentage of alcohol affects the aluminium-morin system
considerably, as a large percentage causes non-linearity in fluorescence quenching. This
method suffers from many interferences by cations and anions, which led to the
suggestion of a method for isolating the phosphate ion. Guyon and Shults [626] also
studied this system and applied it to the determination of phosphate in well and pore
waters. The interfering fluoride ions are removed by boiling the slightly acidic sample
and the cationic interferences by precipitation with hydroxide ions.
Phosphorus can be determined as orthophosphate by the formation of the ion-
association complex of molybdophosphate with the basic dyestuff Rhodamine B [627].
After the extraction of excess of dye reagent into chloroform, the Rhodamine B-
molybdophosphate is extracted into chloroform-butanol (4+1). A study of the
stoichiometry in the ion-association complex revealed a combining ratio of 3mol of
Rhodamine B to 1mol of molybdophosphate. This is in accordance with the formation of
an unchanged complex of the type [RhB
+
]
a-
[PMo
3−
].
The orthophosphate ion is also determined by precipitation of quinine
molybdophosphate in 0.5M sulphuric acid. The excess of the quinine reagent is removed
by washing the precipitate with 0.5M sulphuric acid and the complex is dissolved in the
solvent mixture acetone-0.5M sulphuric acid (9+1) [628]. The authors suggested that the
precipitate corresponds to the composition (C
2
0H
24
O
2
N
2
H
2
)
3
(PMo
1
2O
40
)
2
; similar to
that of quinine-molybdovanadophosphate as proposed by Ripau and Siteu [629].
Wang
et al.
[630] have also described a method based on quenching of Rhodamine 6G
fluorescence for the determination of phosphate in non saline waters.
2.75.3
Flow injection analysis
Janse
et al.
[631] have optimised conditions for the determination of phosphate by flow
injection analysis. The flow rates of the water carrier stream and the reagent streams, the
injection volume and the lengths of coils are the parameters on which the procedure was
optimised.
Motomizu
et al.
[632] determined phosphate in river water by flow injection analysis.

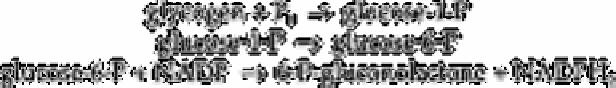
Search WWH ::

Custom Search