Chemistry Reference
In-Depth Information
However in some instances, concentrations of chloride, sulphide and fulvic acid
substances may exceed the above levels. Therefore, it was necessary to incorporate an
alternate method for the removal of these interferences prior to the determination of
nitrate.
Fig. 2.28
Unconnected fluorescence spectra of solutions run through the
manifold In Fig. 2.27. Curve A emission spectrum of DHDMBP;
Curve B emission spectrum of 2× 10
−6
mol L
−1
nitrate+DMBMBP;
Curve C excitation spectrum of 1×l0
−6
mol L
−1
nitrate +DHDMBO;
Curve D emission spectrum of 1×10
−6
mol L
−1
quinine sulphate in
0.1N sulphuric acid. For all spectra, coarse sensitivity=1, fine
sensitivity=80, excitation and emission=2.0. For A and B excitation
for C emission and D excitation
Source: Reproduced with permission from the American Chemical
Society [431]
In the procedure the sample is diluted with mercuric sulphate to avoid interference by
chloride and sulphide. An XAD-2 resin column was incorporated after the sample
dilution step to eliminate interference from fulvic acid substances: ammonia, glycine,
urea, amino acids and nitrite were without interference in the procedure.
Bianthronyl method
An extensive study of the kinetics and mechanism of the reaction between nitrate and
binthronyl in concentrated sulphuric acid has been carried out [432]. Initially, nitrate
reacts with sulphuric acid giving the nitronium cation, which acts on the bianthronyl
molecule:

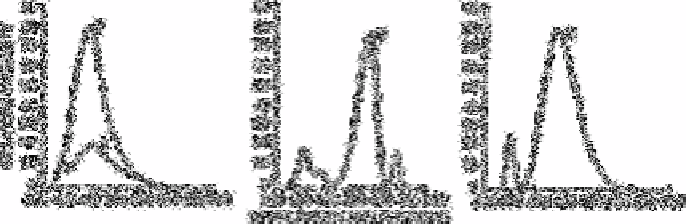
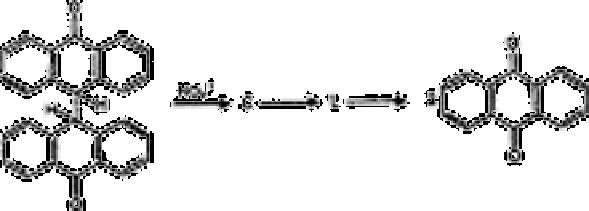
Search WWH ::

Custom Search