Chemistry Reference
In-Depth Information
2.19.2
Flow injection analysis
Ruz
et al.
[221] speciated different oxidation states of chromium. They were able to
obtain a concentration profile for the chromium(III) and chromium(VI) species, namely
HCrO
4
−
, Cr
2
O
7
2−
and CrO4
2
−
. Ruz
et al.
[222] also used reverse flow injection analysis
for the simultaneous and sequential determination of chromate and chromium(III) by
unsegmented flow methods. A photometric detector was employed.
Ruz
et al.
[221] used the flow injection analysis technique together with computerised
numerical calculation methods to study the distribution of the type of water analysed and
the above-mentioned pK values. By adding a microprocessor to the flow injection
analysis system, and creating a suitable calculation program, the different equations
established can be conveniently solved. The occurrence in the medium of complexing
species for chromium, and/or the presence of a colloid resulting in adsorption phenomena
for chromium ions, have not been taken into account. Including these would require
information about the type of ligand and/or colloid, their concentration and corresponding
equilibrium constants; additionally, the calculation program would need to be modified.
The value of the ionic strength of the sample must be taken into account because it
affects the values of the constants. Constants with an ionic strength of 0.1M were used by
Ruz
et al.
[221]. The reversed flow injection analysis, reverse flow injection analysis and
asymmetric merging zones [211] were also utilised, together with the indicator reaction
reported by several authors [207-212] involving Cr(VI) and 1,5-diphenylcarbazide.
Tandon
et al.
[213] have carried out a theoretical study on the effect of the pH on the
Cr(VI) species present in solution. Cr(III) species were not considered.
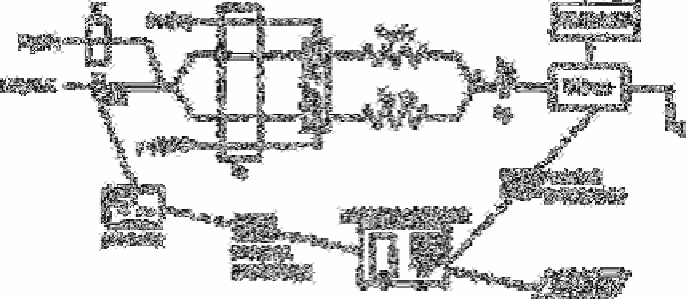
In this procedure [221] the pH of the water sample, which travels through the system,
is continuously monitored (glass-calomel microelectrode-ME- in Fig. 2.15) prior to its
confluence with a sulphuric acid stream, whereupon the stream is split into two channels
with an injection valve each. The oxidant (Ce[IV]) is injected into channel 1, while 1,5-
diphenylcarbazide is injected into channel 2. The reactor lengths and
Fig. 2.15
Diagram of the system used for chromium speciation studies.
Optimum working conditions: diphenyl carbazide 0.17% (w/V)/Ce
(IV)/0.5g L
−1
/H
2
SO
4
/1M, q, 3.28ml min
−1
, q] 0.30ml min
−1
, V
1
Search WWH ::

Custom Search