Chemistry Reference
In-Depth Information
Chromium(III)
The pH range over which each species predominates can be conveniently inferred from
plots of log/concentration/versus pH [215].
2.19.1
Spectrophotometric methods
Aoyama
et al.
[216, 217] have described a rapid method for the determination of down to
3µg L
−1
of chromate in which hexavalent chromium is complexed with
diphenylcarbazide, and this complex is floated with a sodium lauryl sulphate anionic
surfactant. After dilution the subsidised foam is measured spectrophotometrically. The
continuous flotation procedure is as follows.
Place the water (1 L), previously acidified to 0.1mol L
−1
with sulphuric acid, in the
separation tube, pass nitrogen into the tube at about 105ml min
−1
, and pump in the 1%
sodium lauryl sulphate solution at 0.39 ml min
−1
. Continuously supply the sample pH and
the 1% diphenylcarbazide solution to the separation tube at a rate of 2L h
−1
and 0.230ml
min
−1
respectively. A steady state flotation was achieved 1 h after flotation had started.
Mix the sample and diphenylcarbazide solutions in the mixing chambers before entering
the tube. Collect the foam subsided in the collector and intermittently transfer to flasks
and dilute to 50ml with water, and measure absorbances as described above.
The effect of diverse ions on the flotation of chromium(VI) were investigated. Only
copper(II) and iron(III) in 10-fold amounts, and vanadium(V) in 20-fold amounts caused
fading of the colour. Even in those cases, however, chromium(VI) could be measured by
a standard addition method.
Diphenylcarbazone and diphenylcarbazide have been widely used for the
Spectrophotometric determination of chromium [218]. Only relatively recently, however,
has the nature of the complexation reactions been elucidated. Cr(III) reacts with
diphenylcarbazone where Cr(VI) reacts (probably via a redox reaction combined with
complexation) with diphenylcarbazide [219]. Although speciation would seem a likely
prospect with such reactions, commercial diphenylcarbazone is a complex mixture of
several components, including diphenylcarbaizide, diphenylcarbazone,
phenylsemicarbazide and diphenylcarbidiazone with no stoichiometric relationship
between the diphenylcarbazone and diphenylcarbazide [220]. Total chromium can be
determined with diphenylcarbazone following reduction of all chromium to Cr(III).

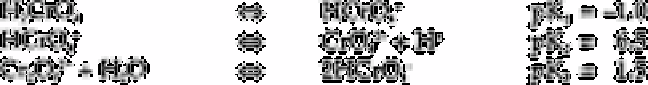

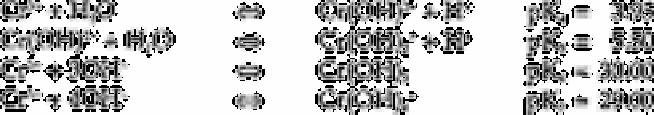
Search WWH ::

Custom Search