Chemistry Reference
In-Depth Information
The bromoform was determined by head space gas chromatography. The calibration
graph was linear for amounts of bromide in the range of 0.3-5.0µg and the detection limit
was 0.1µg. The relative standard deviation was 4.3% for 2.0µg of bromide. Chloride ion
(<1000µg) did not interfere. Aluminium, iron, zinc and humic acid had a positive
interference, and iodide, glutamic acid and glucose had a negative interference.
Ando and Sayato [144] have described a gas chromatographic method for the
determination of bromide ions in water. These workers found that the use of an ion
exchange gas chromatograph using strong anion exchange resins enabled them to
separate and concentrate chloride and bromide. The bromide was eluted with 0.1 mol L
−1
sodium nitrate through a 70mm column, until 70 ml of eluate was obtained (total 130ml).
After elution of chloride with dilute sodium nitrate, the solution of bromide could be
eluted with 0.5 mol L
−1
sodium nitrate. Interfering effects of phenols and humic acids
could also be overcome by these procedures.
Bromide concentrations down to 1-2µg L
−1
can be determined by this procedure.
Calibration curves are linear up to 1.5mg L
−1
bromide.
Nota
et al.
[145] have devised a simple and sensitive method for the determination of
amounts of bromide down to 0.05mg L
−1
in water. The procedure is in two stages.
Cyanogen bromide is formed by the reaction between bromide, chlorine and cyanide.
Cyanogen bromide is then separated by gas chromatography and selectively detected
with an electron capture detector.
It should be noted in this method that the internal standard (nitromethane) must always be
introduced after the addition of cyanide in order to prevent rapid darkening of the
solutions, which causes nonreproducible results. No interferences are caused by oxidising
or reducing substances or by mercury or cadmium at concentrations below 200 mg L
−1
.
Mercury and cadmium are, among the metals, the strongest complex-forming agents with
bromide.
Even small amounts of aromatic compounds are likely to produce some interferences
owing to their tendency to bind bromide, which is formed by reaction with chlorine
water.
The application of gas chromatography is also discussed under multianion analysis in
section 14.1.1.2.

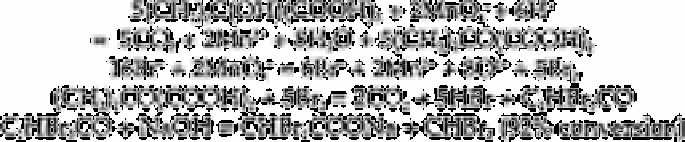

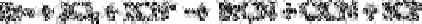
Search WWH ::

Custom Search