Environmental Engineering Reference
In-Depth Information
ρ
ρ
0
0
2
t
*
=
Ck
d
+
CD
d
(2.26)
p
,
0
p
,
0
2
8
Ab s
,
Ab
,
m
where ρ
0
is the net density of particle. Equation 2.26 coincides with the second-
order rule of Nusselt,
51
as shown in the following equation:
ρ
0
2
t
*
=
CD
d
Ab
(2.27)
p
,
0
8
,
m
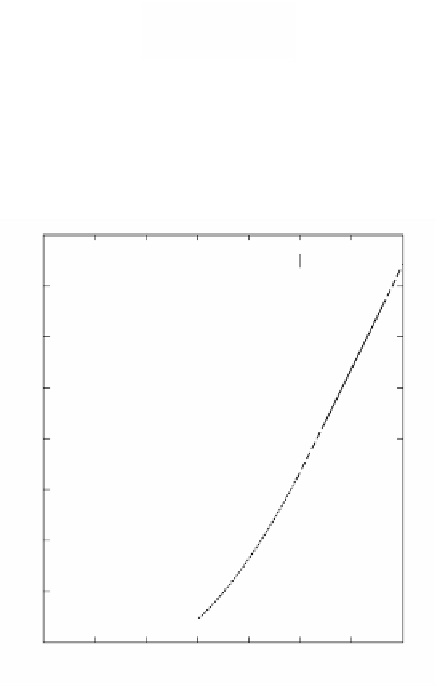
and the diameter of particle. Combustion temperature is 1773 K. The relation
coincides very well with Equation 2.27 in the case of 100-µm particles, which shows
clearly the diffusion defined process.
10
7
Time
sec
captive
p.c.range
particle range
10
6
10
3
Experimental combustion
times of single (captive)
particles (10coals:300
microns to 4m.m.):t
c
d
2
10
5
10
2
10
4
10
10
3
one second
1
Range of experimental
combustion times
in flames
10
2
I.G.Farben (BASF) Data
t
c
d
0
(empirical)
10
0.4
Predicted combustion
times:t
c
=K
c
d
o
+K
D
d
o
(Theoretical Derivation)
1
2
10
-1
10
-3
10
-2
10
-1
0
10
10
2
10
3
10
4
Particle Diameter, Microns
FIGURE 2.94
Relation between complete combustion time and diameter of solid particle.
2.5.2.5 Combustion Behavior of Coal at Synthetic Air
Condition of High Temperature
To clarify the reaction behavior of coal under the condition of high-temperature air,
it is necessary to design a combustion furnace for estimating quantitatively the
emission rate of volatile matter. In this section, an example of the reaction behavior
of coal under the condition where it is actually reacted under synthetic high tem-
perature air is introduced.
This test plant is a horizontal type reaction furnace using pulverized coal under











Search WWH ::

Custom Search