Environmental Engineering Reference
In-Depth Information
in most practical combustion devices, and that significant reductions in NO emissions
are achieved by reducing both local and overall temperature levels.
In Section 2.3.3 we showed that flame temperature in HiTAC varies depending
on the mixing process in the furnace, even if it had the same air excess ratios. Further,
the temperature level is not always high compared with that in ordinary combustion
in spite of the use of preheated combustion air. The primary reason NO emission
was reduced significantly is that the reduced flame temperature resulting from the
dilution of combustion air with the burned product yielded a comparatively distrib-
uted heat release in the low oxygen concentration atmosphere. These characteristics
are studied numerically on a laminar diffusion flame between fuel and air or air
diluted with combustion products in counterflow configuration using a detailed
database of chemistry called CHEMKIN.
26,18
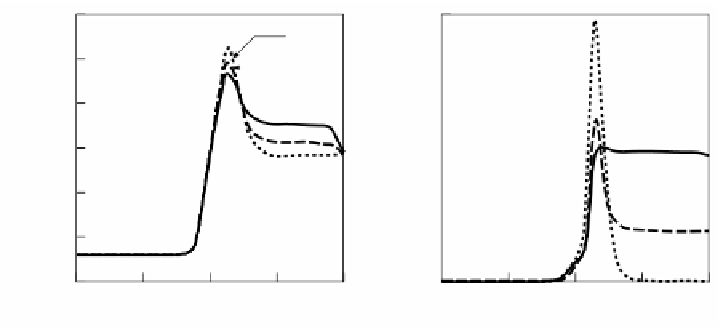
Figure 2.61
shows the variation of temperature distribution and NO concentration
in flames for different preheating of combustion air. The peak temperature increases
with the preheat temperature of the air and results in high concentration of NO.
According to the numerical predictions done by Ju and Niioka,
25
NO is mainly
formed through the thermal mechanism on the high temperature air-side of a flame
and through the prompt mechanism in the narrow flame zone, and the emission
index increases dramatically with preheated air temperature.
25
The influence of oxygen concentration in nitrogen-diluted air on NO formation
is demonstrated in
Figure 2.62
.
The peak temperature in the flame and corresponding
NO concentration show drastic decrease with the oxygen concentration in spite of
the constant preheating temperature of diluted air at 1400 K. In the case of an oxygen
concentration of 10%, the maximum flame temperature falls to almost the same
level as for pure air of ambient temperature. NO concentration far below that of the
non-preheated flame is predicted for an oxygen concentration of 5%. We can see
that the concentration of oxygen is also a dominant factor in NO formation as far
as diffusion combustion occurring in furnaces is concerned.
3000
2500
O = 21%
2
O = 21%
2
2500
1
5%
10%
2000
15%
2000
10%
1500
1500
1000
1000
T = 300K
fuel
500
500
0
0
0
0
10
20
30
40
10
20
30
40
X mm
X mm
FIGURE 2.61
Effect of initial NO concentration in diluted air.

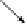
Search WWH ::

Custom Search