Biology Reference
In-Depth Information
expression of APE1 was reduced by 80% (as measured
using Western blotting) and the menadione toxicity
was significantly greater (
Figure 13.2
B). For example,
in cultures treated with scramble siRNA, 10
treated with siRNA also were exposed to adenovirus
containing a human APE1 construct, the resulting over-
expression of APE1 resulted in a significant attenuation
of menadione-induced histone phosphorylation
(
Figure 13.3
C), whereas the adenoviral vector control
had no significant effect (
Figure 13.3
B). Together, these
results and those from other studies provide causal
data showing that altering APE1 expression and
presumably the activity of BER is neuroprotective
against neurotoxicity produced by oxidative DNA
damage.
Two studies directly address the effects of manipu-
lating DNA repair on cisplatin-induced neuronal
damage. Using mice deficient in the NER, Dzagnidze
and co-workers showed that the lack of activity in
this repair pathway correlated with an increased
cisplatin-induced neurotoxicity.
58
They examined the
effects of acute exposure or chronic exposure to
cisplatin in NER repair competent mice (as controls)
and mice deficient in xeroderma pigmentosum comple-
mentation group A (XPA) or xeroderma pigmentosum
complementation group C.
58
Mice were given 2mg/kg
cisplatin i.p. and levels of guanine
M of mena-
m
dione resulted in a 50
3 % loss of cell viability, whereas
in cultures treated with APE1siRNA, cell
loss was
75
4%. Overexpression of APE1 in the cultures attenu-
ated the toxic effects of menadione in cells with normal
APE1 expression (
Figure 13.2
A) or in cells with reduced
APE1 expression (
Figure 13.2
B). In another series of
experiments, we measured the effects of menadione
exposure on the phosphorylation of histone H2AX as
an indicator of DNA damage. In these experiments,
sensory neuronal cultures were exposed to 3
M mena-
dione for 1 hour and the H2AX phosphorylation was
measured prior to exposure and at 2 and 4 hours after
the drug was removed.
Figure 13.3
A illustrates that
menadione significantly increased H2AX phosphoryla-
tion at both 2 and 4 hours after the oxidative stress in
cells treated with scramble siRNA. In cultures treated
with APE1siRNA, which reduced APE1 expression by
85% (as measured by Western blotting): the mena-
dione-induced phosphorylation of H2AX was signifi-
cantly increased. When sensory neuronal cultures
m
guanine intra-
strand cross-links were measured in the spinal cord
e
(A)
(B)
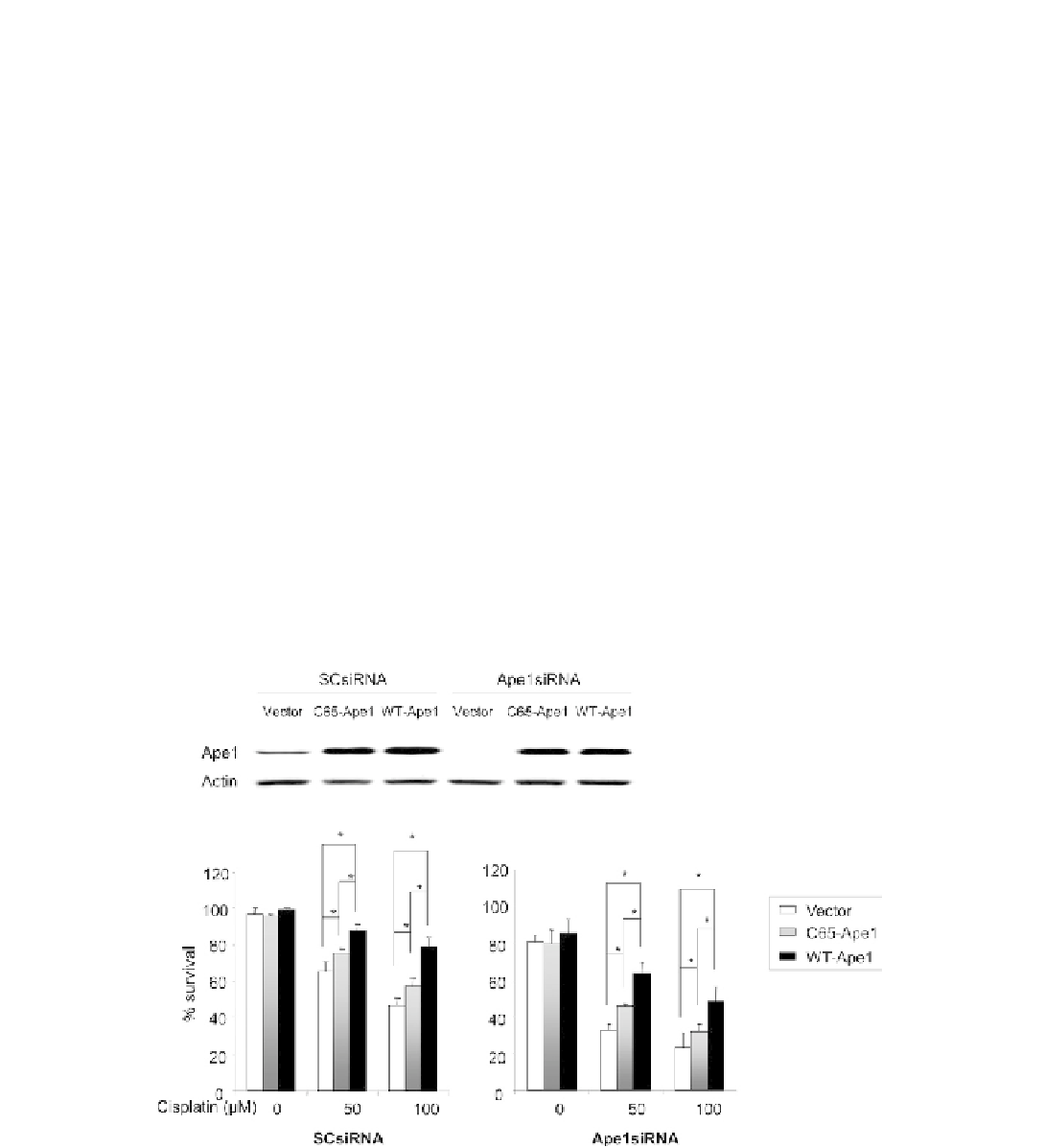
FIGURE 13.4
Cisplatin-induced cell death in sensory neuronal cultures is increased by a reduction in APE1 expression and attenuated by
APE1 overexpression. A: A representative Western blot showing the effects of siRNA treated and adenoviral infection on APE1 expression in
sensory neuronal cultures. Sensory neuronal cells treated with 100 nM of scramble siRNA (SCsiRNA) or APE1siRNA on day 4
6 in culture and
infected with one of three adenoviral constructs; vector control, WT-APE1or C65-APE1 on day eight for 24 hr as indicated. The protein was
extracted on day 12 of culture and analysis performed. B: The ordinate represents the mean
e
SEM of the percent of cells surviving at 24 hr as
measured by trypan blue exclusion from three independent harvests after cultures were exposed to different concentrations of cisplatin for 24 hr.
Neuronal cultures were pretreated with scramble siRNA (SCsiRNA) or APE1siRNA as indicated. The open columns represent cultures infected
with the vector control, the lightly shaded columns are cell infected with adenoviral constructs containing the C65 mutated APE1 and the dark
columns are cells infected with virus to APE1. An asterisk indicates a statistically significant difference in cultures infected with the viral vector
versus exposure to virus containing APE1 constructs using analysis of variance and the Tukey post-hoc test. From Jiang et al.,
10
with permission.