Environmental Engineering Reference
In-Depth Information
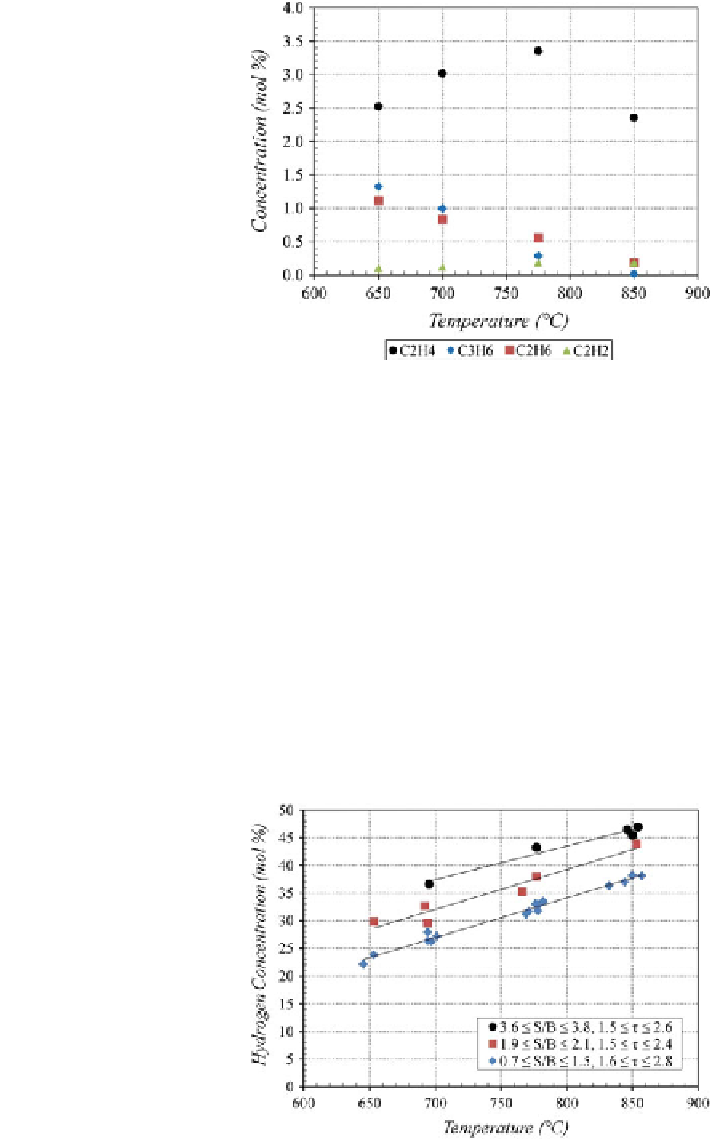
Fig. 8 Effect of temperature
on higher hydrocarbon
concentrations
(S/B = 2.0
±
0.1,
τ
= 2.0
±
0.4 s)
temperature. The hydrocarbons crack at high temperatures to produce lighter
molecular weight compounds.
The trend of increasing hydrogen concentration with increased temperature held
for all S/B ratios and residence times tested. Figure
9
shows the similar rate of
increase in hydrogen concentration for three different bed conditions. Hydrogen
concentration increased by an average of 7 mol% per 100
C increase in reactor
temperature. The hydrogen concentration increased due to the increase in gas-phase
steady-state concentration as well as increased char reactivity. The char
-
steam
gasi
°
cation reaction is endothermic and occurs at very low rates at low tempera-
tures. The results presented are in agreement with published data (Franco et al.
2003
; Koppatz et al.
2011
). A hydrogen concentration maximum was not found in
the present experimental study, and an increase in temperature would almost
certainly increase hydrogen concentration further. In order to achieve an average
bed temperature of 900
°
C, the reactor exterior temperature in the main heating
Fig. 9 Effect of temperature
on hydrogen concentration for
different S/B and
τ
Search WWH ::

Custom Search