Environmental Engineering Reference
In-Depth Information
increased CGE with increased temperature holds for all of the facilities. The CGE
found in the present study matches (within limits of error) the results obtained by
Franco et al. at temperature above 750
°
C. The agreement in the CGE values
con
rms proper operation of the gasi
er energy measurement systems.
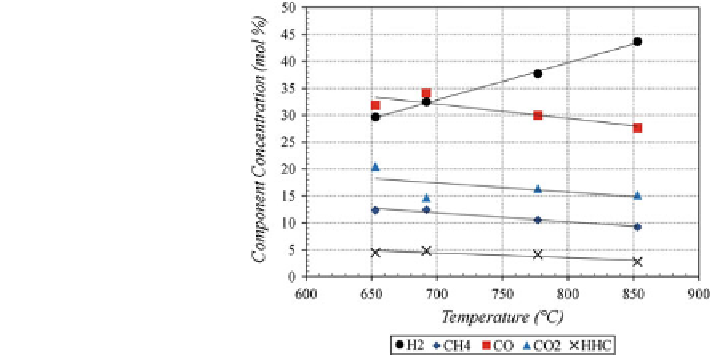
3.1 Syngas Composition
The effect of the reactor temperature on syngas composition was studied between
650 and 850
C. Figure
7
shows the concentration of the major syngas constituents
for moderate S/B ratios and residence times. At 650
°
C, carbon monoxide was the
most dominant syngas component at 32 mol%, followed closely by hydrogen at
30 mol%. When the reactor temperature was raised, the hydrogen concentration
increased, while all of the other constituents gradually decreased. At 775
°
C,
hydrogen was the most dominant species at 38 mol% and carbon monoxide levels
had dropped to 30 mol%. This trend continued when the reactor temperature was
raised to 850
°
C, where a hydrogen concentration of 44 mol% was recorded. Part of
the reason for the increase in hydrogen concentration was the cracking of hydro-
carbons. Methane levels dropped from 13 to 10 mol%, and higher hydrocarbon
(HHC) levels dropped from 5 to 3 mol%.
The effect of temperature on HHC concentration is shown in Fig.
8
. Ethylene
(C
2
H
4
) levels increased as the reactor temperature was raised from 650 to 775
°
°
C
before falling to its lowest level at 850
C. The increase in ethylene concentration
could have been from the cracking of propylene (C
3
H
6
). Propylene contains two
carbon atoms joined by a double bond (like ethylene) and with a methyl (methane)
group attached to it. All of the other HHCs decreased in concentration with each
subsequent increase in temperature. The resulting sum of the HHC
°
'
s decreased with
Fig. 7 Effect of temperature
on gas composition (S/
B = 2.0
±
0.1,
τ
= 2.0
±
0.4 s)
Search WWH ::

Custom Search