Biomedical Engineering Reference
In-Depth Information
Pleural space
PDGF
CCL2
Mononuclear cell
aggregate
Mesothelium
Collagen
Pleura
Subpleural
brosis
Lymphatic duct
Fibroblasts
Alveolus
PDGF,
TGF-b1
Macrophage
Type I
epithelium
Inhaled carbon nanotubes
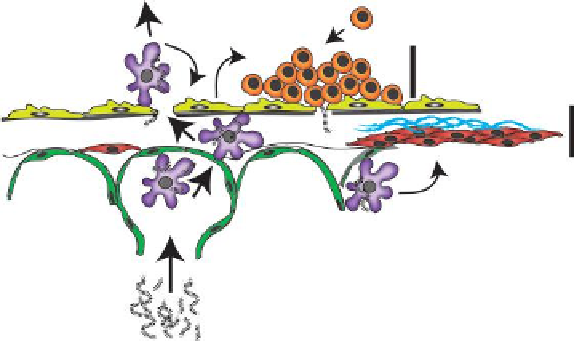
FIGURE 10.5
Illustration depicting migration of inhaled MWCNTs to the pleural lining
surrounding the lungs and resulting inflammatory and fibrotic response.
et al. produced mononuclear cell accumulation at the pleural surface within 1 day
after exposure and subpleural fibrotic lesions within 2 weeks, which resolved by 6
and 14 weeks, respectively. MWCNTs delivered to mice penetrate the pleural lin-
ing (Mercer et al. 2010). However, no evidence of mesothelioma has been observed
in mice exposed to CNTs by any method, with the exception of genetically sus-
ceptible p53-deficient mice. This could be due to the fact that mice do not read-
ily develop mesothelioma, even in response to known agents (e.g., asbestos) that
cause mesothelioma in humans. The issue of whether CNTs are capable of causing
mesothelioma in humans remains a key topic of research that will have important
implications for responsible use of CNTs. So far it is unknown whether CNTs repre-
sent a new cancer risk factor for humans. However, a recent report by NIOSH set a
recommended exposure limit for MWCNT based on rodent studies (Castranova et al.
2012). Although CNTs cause lung fibrotic reactions in the interstitium and pleura of
mice, their carcinogenic potential has not been adequately addressed. Longer term,
low dose studies with CNTs will need to be undertaken to adequately address the
potential carcinogenicity of CNTs. Elucidating the carcinogenic potential of CNTs
at the pleural lining in rodents using relevant inhalation exposures with appropriate
positive controls (e.g., asbestos fibers) will have important implications for the future
use and development of CNTs for a variety of applications.
Several studies show that CNTs have the potential to cause genotoxic effects in
lung cell types and in rodents
in vivo
. Work by Sargent et al showed that SWCNTs
caused fragmented centrosomes, mitotic spindle disruption, anaphase bridges, and
aneuploid chromosome number in cultured primary or immortalized human airway
epithelial cell types (Sargent et al. 2009, Sargent et al. 2012). These studies showed
disruption of the mitotic spindle by SWCNT and the authors noted that the similar
Search WWH ::

Custom Search