Biomedical Engineering Reference
In-Depth Information
Pl
Pl
Al
20
µ
m
Al
(a)
2
µ
m
Pl
(c)
Al
50
µ
m
100
nm
(b)
(d)
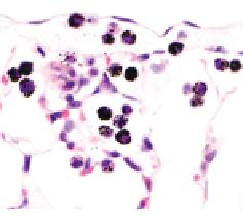
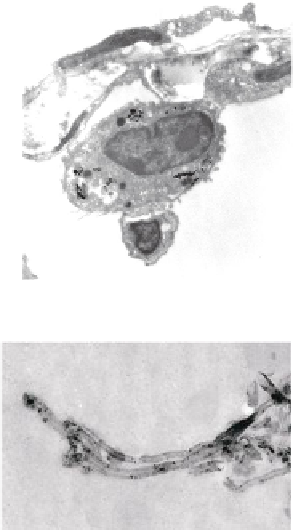
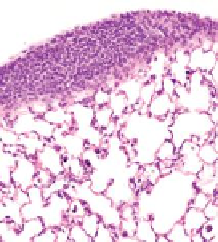
FIGURE 10.4
Multiwalled carbon nanotubes (MWCNTs) within the subpleural tissue
after inhalation exposure in mice. (a) Light microscopy of macrophages with MWCNTs
(arrows) abundant in the subpleural tissue and (b) associated with the formation of pleu-
ral (Pl) mononuclear cell aggregates (arrowheads). (c) and (d) Electron micrographs of
MWCNTs within subpleural macrophage (arrow). (Images in (a), (b), (c) and (d) reprinted
by permission from Macmillan Publishers Ltd.,
Nat Nanotechnol
, Ryman-Rasmussen
J.P. et al. Inhaled multi-walled carbon nanotubes reach the subpleural tissue in mice. 4:
747-751, copyright 2013.)
(Ryman-Rasmussen et al. 2009b). As shown in Figure 10.5, fibrosis at the sub-
pleural region could be driven by growth factors, PDGF and TGF-β1, produced by
MWCNT-stimulated macrophages.
Injection of long MWCNTs into the peritoneal cavity of mice (a surrogate for the
pleura) induces inflammation and granuloma formation, suggesting that MWCNTs
have asbestos-like pathogenicity (Poland et al. 2008). Also, p53 heterozygous null
mice, which are susceptible to the development of mesothelioma, had increased
incidence of mesothelioma in the abdominal cavity after intraperitoneal injection of
MWCNTs (Takagi et al. 2008).
MWCNTs delivered to the lungs of mice by nose-only inhalation exposure (Ryman-
Rasmussen et al. 2009a) or by OPA (Porter et al. 2010) demonstrated migration of
CNTs to the pleura. The highest dose used in experiments by Ryman-Rasmussen







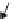
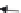
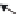






Search WWH ::

Custom Search