Biology Reference
In-Depth Information
1.1 Molecular Structure Determination of GFP
An early breakthrough in GFP research was the successful crystallisation and
structure solution. Very close together in time the structures of the S65T mutant
[
4
], in which the anionic chromophore is stabilised, and the wild-type GFP [
6
], with
a predominantly neutral chromophore, were reported. Interestingly, the first crystals
of the native GFP isolated from
A. victoria
jellyfish were already reported in 1974
[
11
], and X-ray diffraction was recorded in 1988 [
12
]. However, it took many years
before its structure was finally solved, using multiple isomorphous replacement and
anomalous scattering [
4
,
6
] and molecular replacement [
5
]. The results showed that
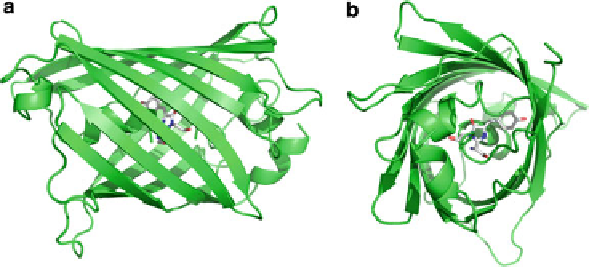
GFP has a beta-barrel structure consisting of 11 beta strands with the light absorb-
ing
p
-hydroxybenzylidene-imidazolidinone chromophore fully embedded within
its core (Fig.
1
). The availability of the structural coordinates started the rational
design of directed mutations that alter the spectroscopic properties.
GFP exists predominantly in the neutral phenol state, with the phenolic
p
-hydroxybenzylidene-imidazolidinone chromophore protonated. The dominant
but partial stabilisation of this species GFP
A
(l
max
¼
398 nm) at pH 8.0 results
from electrostatic repulsion by an acidic group, identified from X-ray crystallogra-
phy to be the carboxylate of Glu222 [
5
,
6
]. The thermal equilibrium properties of
proton transfer reactions include a plateau in the pH titration in the pH 7-10 region,
which is predicted from a four-state system that describes the interaction of the two
ionisable groups [
13
]. A comparison of the molecular interactions in the chromo-
phore region of the wild-type (Fig.
2a
) and the S65T mutant (Fig.
2b
) highlights the
stabilised neutral GFP
A
and anionic GFP
B
states, respectively. The stabilisation of
the neutral and anionic forms in these proteins is central to understanding the
spectroscopic details as well as the details of the photoconversion and the fluores-
cence reactions, which will be reviewed.
Fig. 1 (a) Crystal structure of the green fluorescent protein, drawn from 1GFL.pdb [
6
]. (a) A “side
view” of the secondary structure cartoon representation and (b) “end-on view”, showing the
p
-hydroxybenzylidene-imidazolidinone chromophore buried inside the beta-barrel
Search WWH ::

Custom Search