Biology Reference
In-Depth Information
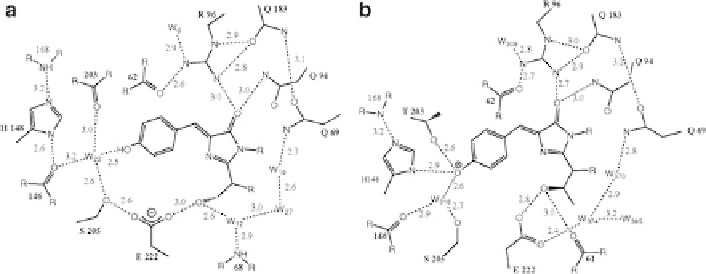
Fig. 2 Comparison of the chromophore environment of the wild-type (a) and the S65T mutant (b)
of GFP. Reproduced with permission from [
5
]. In the wild type (a), the phenol group is predomi-
nantly protonated, identified as the GFP
A
(l
max
398 nm) state. In the S65T mutant (b), the H-
bonding network is different such that in contrast to the wild type the acidic Glu222 side chain is
not in indirect H-bonding contact with the phenolic oxygen, which leads to stabilisation of the
anionic GFP
B
(l
max
¼
¼
476 nm) state
1.2 The Fluorescence Photocycle of GFP
It has been known since the early purification and characterisation of native GFP
from jellyfish that fluorescence emission with blue excitation is green, and hence
that the Stokes shift for the GFP
A
state is very large, in contrast to that of the GFP
B
state [
11
,
14
,
15
]. The absorption spectrum of the wild-type GFP shows peaks at
280, 398 and 476 nm, which are also observed in the fluorescence excitation
spectrum (Fig.
3
). The maxima at 398 and 476 nm belong to the neutral and the
anionic chromophore, respectively, indicating that at pH 8.0 two species exist, the
neutral form being the dominant population. The amplitude at 398 nm has about
three times the amplitude of the 476 nm maximum, but the latter is associated with
an extinction coefficient that is twice that of the neutral form. Therefore, the molar
ratio of neutral to anionic chromophores is approximately 6:1 [
16
]. A structural
basis for this heterogeneity was proposed by Sixma and co-workers [
5
]. It was noted
that the neutral form is stabilised by indirect electrostatic repulsion from the acidic
carboxylate group of Glu222, via an H-bonding network including Ser205 and a
crystallographic water molecule (Fig.
2b
). The green emission with blue excitation
of the GFP
A
neutral state is illustrated in Fig.
3
, which indicates with double arrows
both the Stokes shifts for the GFP
A
and GFP
B
states at room temperature (Fig.
3
).
While the fluorescence emits from the radiative deprotonated species (GFP
I
*) in
both cases, the species formed with GFP
A
excitation is a more relaxed form (Fig.
3
).
Pivotal work by Steven Boxer in 1996 studied the ultrafast fluorescence spec-
troscopy of the GFP
A
state, which led to the discovery of excited state proton
transfer (ESPT) in GFP [
16
]. Chattoraj et al. performed fluorescence upconversion
measurements to show that after blue (400 nm) excitation of GFP
A
, emission from
the excited neutral species GFP
A
* detected at 460 nm interconverts on picosecond
Search WWH ::

Custom Search