Environmental Engineering Reference
In-Depth Information
1977
1988
2000
2009
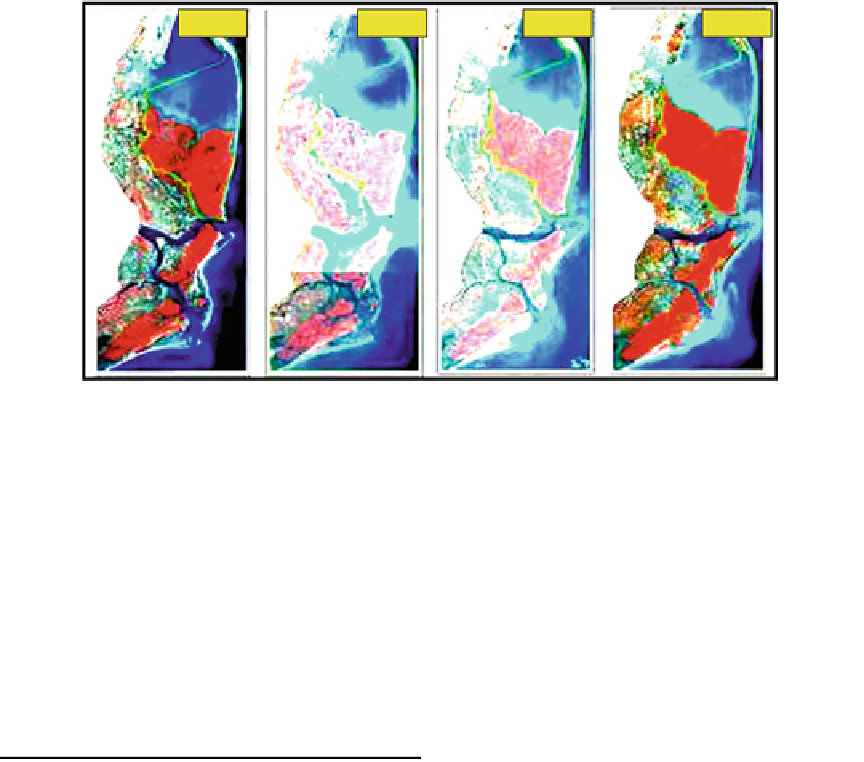
Fig. 3.7
Multitemporal false colour composite images of Godavari mangroves
aquaculture (represented by sky blue colour),
mud
carbon determinations of both the solids left after
acidi
ats (represented by dull blue colour),
plantation (represented by brownish tinge),
agriculture (represented by yellow colour) and
built-up area (represented by pink colour) with
water surrounding the zone (deep blue colour)
(Fig.
3.8
). The aerial extent of vegetation and
other land cover in the Godavari mangroves is
represented in Table
3.3
.
fl
cation with an elemental (CHN) analyser
and the spent acid solution with a dissolved
organic carbon (DOC) analyser.
A more recently developed method (Weliky
et al.
1983
) also discriminates inorganic carbon
from organic carbon by acidi
cation, but has the
advantage that both forms are determined
sequentially in the same sample using only one
instrument. The sample is weighed into a small
fl
ask connected in line to a molecular sieve car-
bon dioxide trap and a carbon analyser. Inorganic
carbon is quanti
3.2
Chemical Method
cation with H
3
PO
4
and boiling to evolve carbon dioxide. The
residual sediment is then boiled with acidic
dichromate solution to evolve organic carbon as
carbon dioxide. This procedure is applicable to
sediments of widely varying composition
including calcareous oozes that contain high
levels of acid-soluble organic matter (Froelich
1980
).
Both of these procedures provide substantial
improvements in accuracy. However, Froelich
ed by acidi
In 1980, Froelich showed that organic carbon
and inorganic carbon cannot be quanti
ed with
high accuracy and precision by difference upon
combustion methods because the thermal
decomposition ranges of naturally occurring
organic carbon and inorganic carbon overlap. It
was also observed by Froelich that when sedi-
ments are treated with aqueous acid to remove
inorganic carbon, from 5 to 45 % of the original
organic carbon can be solubilized and lost (or
counted as inorganic carbon) if the spent acid is
discarded without analysis. To overcome these
problems, he devised an alternate analytical
method for organic carbon that accomplishes
selective removal of inorganic carbon by acidi-
s
procedure is laborious and requires both a DOC
and solid carbon analyser. The method of Weliky
et al. (
1983
) relies on wet oxidation for organic
carbon analysis, a technique which is slower and
not as dependably complete as high temperature
combustion. In addition, total nitrogen values are
not obtained. Both procedures require relatively
'
cation, but accounts for organic carbon lost by
dissolution. This procedure requires organic