Agriculture Reference
In-Depth Information
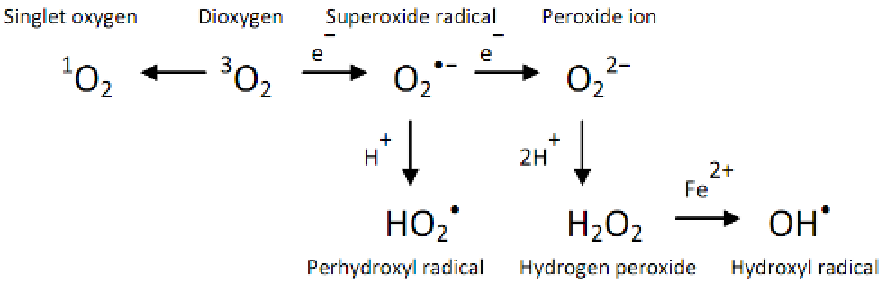
Figure 3.
Formation of different ROS (according to Gill and Tuteja, 2010) [66].
Very reactive
1
O
2
can be formed by the reaction of O
2
with photo-excited chlorophyll. Inade‐
quate dissipation of excess energy during photosynthesis can lead to the generation of chlor‐
ophyll triplet state. The Chl triplet state can react with
3
O
2
to yield
1
O
2
[67]. The formation of
1
O
2
has extremely damaging effect on PSI and PSII and other components of photosynthetic
machinery. The
1
O
2
lifetime has been measured to be approximately 3μs [68]. During this
time, some part of
1
O
2
fraction is able to diffuse over several hundred nm, reacting with pro‐
teins, pigments, nucleic acids and lipids [69]. In
Arabidopsis
mutants favoring
1
O
2
produc‐
tion, photooxidative stress has been demonstrated to cause dramatic increase in lipid
peroxidation (LPO) that precedes cell death [70]. Plant chloroplasts avoid the accumulation
of
1
O
2
by employing β-carotene, tocopherol and plastoquinone to remove it. If the elimina‐
tion is not sufficient,
1
O
2
can lead to the upregulation of genes involved in defense responses
against photooxdative stress [69]. These genes have proven to be different from those in‐
duced by O
2
•−
and H
2
O
2
and it has been suggested that
1
O
2
acts as a signal molecule that acti‐
vates several stress-responsive pathways [71]. The reaction of superoxide radical reduction
produces hydrogen peroxide. H
2
O
2
is moderately reactive but has relatively long lifetime
(1ms) in comparison to other ROS. H
2
O
2
has been shown to be required in a broad range of
physiological processes such as stomata movements [72], cell cycle [73] as well as growth
and development [74]. It plays a dual role. At low, physiological concentration, H
2
O
2
acts as
a signal molecule involved in defense and acclimatory responses to various biotic and abio‐
tic stimuli. At high concentrations, it reacts with cell constituents, e.g. inactivates enzymes
by oxidizing their thiol groups and triggers PCD [75]. The highly reactive ROS - hydroxyl
radical, produced in Haber-Weiss reaction can potentially react with many organic com‐
pounds including DNA, proteins and lipids. Due to the absence of enzymatic mechanism
for
•
OH elimination, its excess production leads to cell death [76].
It has been estimated that 1 - 2% of O
2
consumed by plants is sidetracked for ROS produc‐
tion in different subcellular compartments [66]. Organelles such as chloroplasts, mitochon‐
dria or peroxisomes are major sources of ROS in plant cells since they exhibit an intense rate
of electron flow and highly oxidizing metabolic activity. In chloroplasts, PSI and PSII are
major sites where the singlet oxygen and superoxide radicals are generated. Under non-
stress conditions, the electron from excited photosystem is transferred to NADP
+
, reducing it
Search WWH ::

Custom Search