Agriculture Reference
In-Depth Information
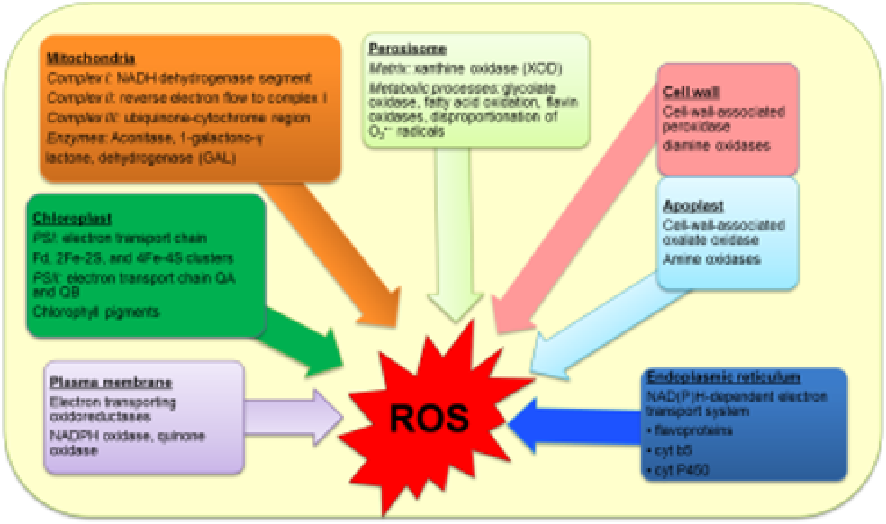
peroxisomes [160, 168; Fig. 4]. But the chloroplast is considered as the main source of ROS in
plants.
Figure 4.
Sites of production of reactive oxygen species (ROS) in plants
Superoxide radical (O
2
●-
) is formed in many photooxidation reactions (flavoprotein, redox
cycling), Mehler reaction in chloroplasts, mitochondrial ETCs reactions, glyoxisomal photo‐
respiration, NADPH oxidase in plasma membranes and xanthine oxidase and membrane
polypeptides. Hydroxyl radical (OH
●
) is formed due to the reaction of H
2
O
2
with O
2
●-
(Haber-
Weiss reaction), reactions of H
2
O
2
with Fe
2+
(Fenton reaction) and decomposition of O
3
in
apoplastic space [169, 170]. Hydroxyl radicals (OH
●
) can potentially react with all biomolecules
like, pigments, proteins, lipids and DNA, and almost with all constituent of cells. Hydroxyl
radical is not considered to have signaling function although the products of its reactions can
elicit signaling responses, and cells sequester the catalytic metals to metallochaperones
efficiently avoiding OH
●
[169, 170]. Singlet oxygen (
1
O
2
) is formed during photoinhibition, and
PS II electron transfer reactions in chloroplasts. This radical directly oxidizes protein, polyun‐
saturated fatty acids, and DNA [171, 172].
The main effects of ROS include autocatalytic peroxidation of membrane lipids and pigments,
modification of membrane permeability and functions [3, 173]. During the time of temperature
stress, ROS level can increase dramatically which can result in significant damage to cell
structure [174]. Vallelian-Bindschedler et al. [175] reported that even very short heat pulses
can result in oxidative bursts of O
2
•-
and/or H
2
O
2
. Heat stress may disturb the homeostatic
balance of cell and promote lipid peroxidation, either by increasing the production of reactive
Search WWH ::

Custom Search