what-when-how
In Depth Tutorials and Information
will be equivalent to when it is expressed during
embryogenesis.
●
How to deliver normal cells to bone? Is it possible to
achieve osteoblast engraftment of bone using the same
approaches used for bone marrow transplantation
or will transplantation require local delivery to each
bone? If they initially engraft, will they become widely
distributed throughout the bone and have sufficient
progenitor potential to maintain the high level of
new bone formation needed to completely remodel
the skeleton with a normal matrix? Stated more
directly, will the engrafted bone show a significant
improvement in its structural properties?
●
Safety. Should therapy be limited to allografts
derived from normal individuals or autographs that
have been genetically corrected? Will genetically
engineered cells or cell that have been extensively
manipulated
in vitro
have unforeseen off-target
effects? How can the spread of cells outside of bone
forming tissues be detected and can they have
biological consequences?
very low and probably below a level to expect a clinical
impact. This mode of therapy has not gained acceptance
by the majority of clinicians who treat children with OI.
Mouse has been the most widely used experimental
animal to test transplant protocols because of the avail-
ability of genetically based visual tools for interpreting
outcomes of a transplantation study. Mice carrying a GFP
reporter, whose expression is restricted to fully differen-
tiated osteoblasts,
67,68
are useful for assessing whether
a transplanted cell population, which does not express
the reporter at the time of transplantation, gains expres-
sion when located on the surface of bone. When GFP
reporters that are not osteoblast specific are employed,
GFP positive cells can be identified on the bone sur-
face,
69-71
but it cannot be assumed that these cells are
osteoblasts. Independent confirmation needs to be used,
which can be the presence of active mineralization lines
from a recent alizarin complexone, calcein or tetracy-
cline injection beneath the GFP cell (
Figure 57.4
), and
strong alkaline phosphatase activity as well as immuno-
histochemical expression of osteoblast-specific proteins
localized to the GFP-positive cell. Using these crite-
ria, a murine bone marrow transplant does not engraft
These are some of the important issues that have to be
considered as new therapeutic strategies are developed
in basic research laboratories and appreciated by clini-
cians who discuss optimistic press reports on the topic of
cell and gene therapy for diseases of the skeleton.
TISSUE SOURCE FOR CELL
THERAPY OF OI
Adult Tissues
Autograph transplantation is highly effective when
used for spinal fusion or non-union because the bone frag-
ments carry with them the same progenitor/osteogenic
potential as in the donating site.
64
Unfortunately, this strat-
egy would not be very effective for the OI subject because
the newly formed bone will be no better than what is
being replaced. Allografts of living bone from a normal
subject are equally unattractive because of immune rejec-
tion problems. For this and many other obvious reasons,
the starting cell source needs to be a cell suspension that
can be delivered either systemically or locally and has the
ability to differentiate into bone after engraftment. Bone
marrow is considered the most clinically relevant source
since it generates the bone that forms trabecular and endo-
cortical bone in the adult individual.
Whole bone marrow was first used clinically in the
mid-1990s as a standard marrow transplant in three chil-
dren with severe OI using normal sibling donors.
65,66
A transient decrease in fracture frequency and accel-
eration in growth was reported but no long-lasting
improvements were observed. The degree of engraft-
ment, as judged by sex chromosome mosaicism, was
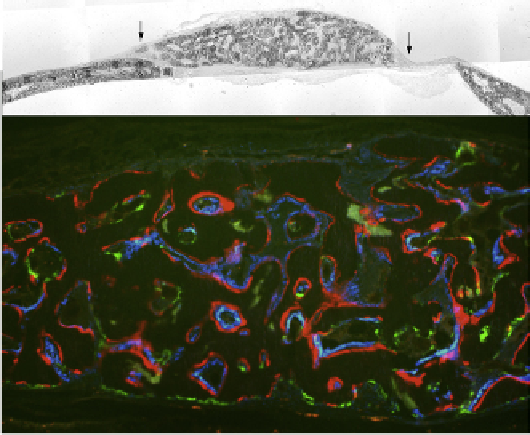
FIGURE 57.4
Use of GFP reporters and fluorescent histological
stains to interpret a bone transplantation study. Using the mouse cal-
varial defect model (
Figure 57.2
), a mixture of BMSCs from a mouse
carrying a blue GFP reporter is mixed with fresh marrow from a mouse
carrying a green GFP reporter. The bone that is formed (upper panel,
between arrows) is examined under fluorescence (lower panel). The
red line is a stain formed by alizarin complexone injected 1 day prior
to sacrifice. It labels actively forming bone surfaces similar to tetracy-
cline. Note that the blue reporter overlies the red label while the green
in most cases lacks an underlying red label. The green cells are mostly
TRAP positive (not shown). Thus the combination of the blue cells from
the BMSC overlying the red label lead to the conclusion that they are an
osteogenic population while the green cells from the fresh bone mar-
row do not make bone although they can be found on the bone surface.