what-when-how
In Depth Tutorials and Information
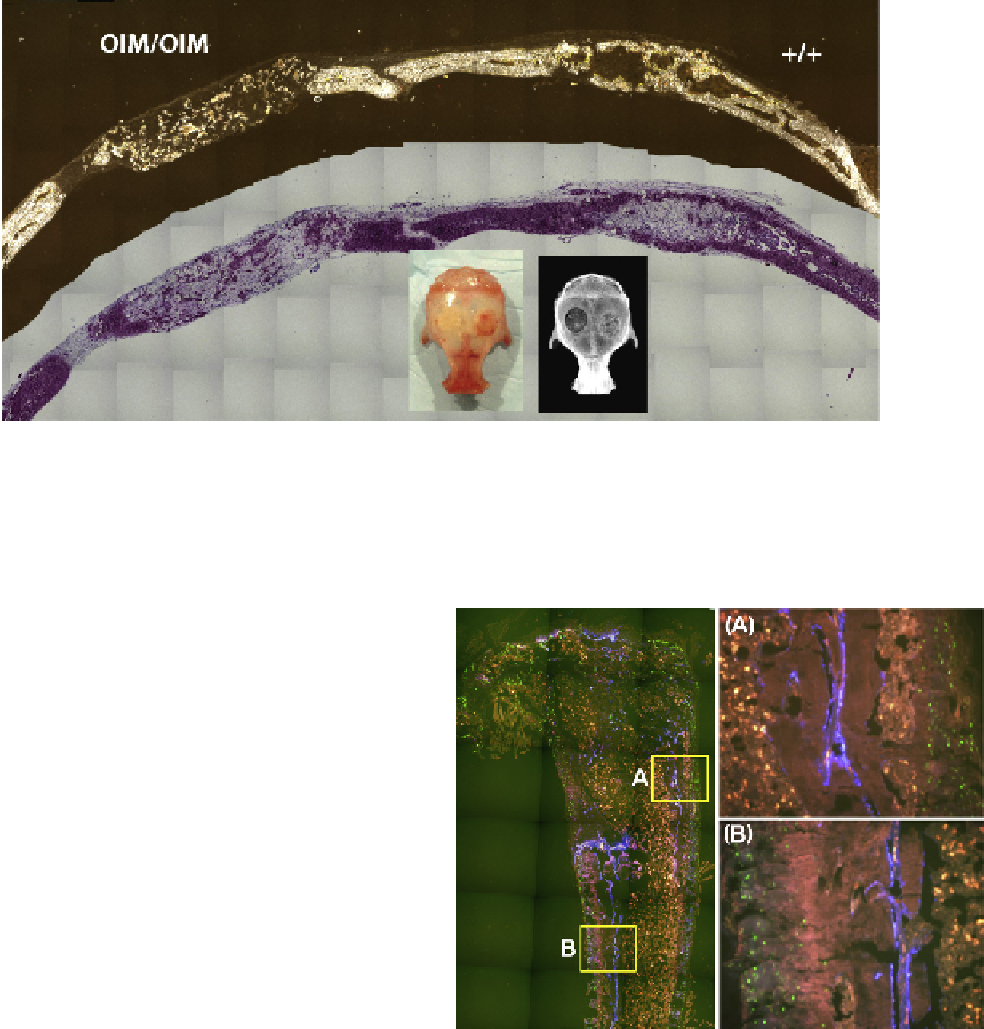
FIGURE 57.2
Osteogenic potential of OI vs. normal osteoblasts in a non-load-bearing bone repair defect. The murine calvarial defect is
a standard method for evaluating the osteogenic potential of a bone progenitor population, which in this case is primary bone marrow stro-
mal cells (BMSCs). A circular defect is created in both sides of the calvaria and filled with a scaffold that is infused with test progenitor cells.
A competent progenitor population (+/+) will make a cortical bone like structure that will bridge the defect zone and integrate with host bone.
However, cells derived from an OI carrying cell source (OIM/OIM) do make some bone but it is sparse and very disorganized.
and have limited ability for full osteoblast differentia-
tion.
26,60,61
In vivo
, the OI bone cells compensate for this
inefficiency by dramatically expanding the progenitor
pool of cells thus providing sufficient mature osteoblasts
to survive.
61
Presumably the stimulus for the expansion
of the progenitor pool comes from the continuous bone-
loading signals of the frail skeleton that cannot provide
adequate resistance to mechanical loading (
Figure 57.2
).
However, in the mosaic individual, the normal cell pop-
ulation will respond to a transient signal for new bone
matrix production and will satisfy the need for increased
structural properties. During that period of stimulation,
the OI population will not be able to expand in number
or make a meaningful contribution to the extracellular
matrix. Thus throughout the period of multiple cycles
of bone modeling and remodeling, the normal mature
osteoblastic cells gradually outpopulate and outproduce
the OI bone cells. The mutation is still present in non-
osteoblastic cells but these cells do not contribute to the
formation of the bone matrix.
This rationale suggests that if it were possible to intro-
duce normal osteoblasts into the bones of an OI subject,
essentially creating somatic mosaicism for the OI muta-
tion, the severity of the bone disease would gradually
diminish as the new cells expanded (
Figure 57.3
) and out-
competed the activity of the resident OI osteoblasts.
62,63
However, there are many unknowns that need to be
answered before this can be considered as a viable option.
FIGURE 57.3
Normal osteoblasts will gradually replace OI bone
when engrafted into the marrow space. Left panel: Primary BMSCs
from a normal mouse carrying a blue GFP reporter construct are
transplanted into the marrow space of an OIM mouse carrying a
green GFP reporter. Blue bone cell develops along the needle tract,
some of which develops into new trabecular bone (A) and some can
become incorporated into cortical bone (B). If better dispersal of the
progenitor cells can be obtained, then improved mechanical proper-
ties of the injected bone might be anticipated.
subjects that are mosaic have been evaluated at a
single time point making it difficult to answer the
question directly. But more important is whether
establishing mosaicism after the skeleton is formed
●
What constitutes success? The critical proportion
of normal cells needed to effectively outcompete
the OI cells is unknown. Only a few human