D. Carriers of Single-Carbon Compounds, and Other Roles of Pterin Coenzymes
The three coenzymes biotin, tetrahydrofolate, and the vitamin Bi2 derivative methylcobalamin (Fig. 7) act as carriers of the single-carbon compounds CO2, bicarbonate ions, formaldehyde, and formic acid. The combining of biotin with CO2 is not a spontaneous process but depends upon adenosine triphosphate (ATP), which serves as both a phospho group carrier and the common energy currency for many cellular reactions. It can also be regarded as a coenzyme. In order to be activated by reaction with ATP, the CO2 must first combine with a hydroxide ion to form bicarbonate HCO- . ATP then transfers a phospho group to the bicarbonate, forming the labile and short-lived car-boxyl phosphate (-OOC—O—PO^) together with adenosine diphosphate (ADP). The carboxyl phosphate, in turn, transfers the carboxyl group to the biotin prosthetic groups of the various carboxylase proteins. From them the car-boxyl group is transferred onto the various sites marked by arrows in Fig. 17. An inorganic phosphate ion is released when the carboxyl group is transferred to biotin, completing a sequence that couples activation of CO2 with the cleavage of ATP to ADP and inorganic phosphate (Pi). Such coupling of ATP cleavage to biosynthesis is a common feature of much of biosynthetic metabolism.
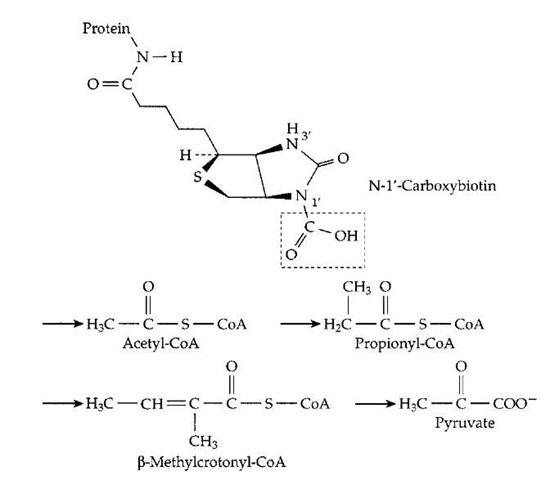
FIGURE 17 The carboxyl carrier function of biotin. A m olecule of activated CO2 is carried as —COOH bonded to N-1′ of biotin, which is covalently attached (as in Fig. 11) to an appropriate protein. Below this structure the sites of four different metabolic intermediates that receive activated CO2 from carboxybiotin are marked by arrows. In each case, either the thioester linkage to coenzyme A or another adjacent carbonyl group activates a hydrogen atom which dissociates as H+, leaving a negatively charged site which accepts the CO2 by direct transfer from carboxybi-otin. Carboxylation of propionyl-CoA in the human body is an essential step in degradation of branched chain and odd chain-length fatty acids (Fig. 12). The resulting methylmalonyl-CoA is converted to succinyl-CoA, the reverse of the reaction shown in Fig. 16.
Two other biotin-dependent reactions of great significance are the carboxylation of acetyl-CoA to malonyl-CoA and that of pyruvate to oxaloacetate (Fig. 17). The former is essential to biosynthesis of fatty acids, which are formed in a pathway which parallels (in reverse) that of j oxidation (Fig. 12). However, there are several differences. In the biosynthetic pathway, acetyl-CoA is first converted to malonyl-CoA which undergoes decarboxyla-tion when a two-carbon unit is added to the growing fatty acid chain. This decarboxylation, together with the prior carboxylation steps, couples ATP cleavage to the biosynthesis. Furthermore, NADPH is used in the reduction steps rather than NADH or FADH2. In addition, the acyl carrier is not coenzyme A but the related prosthetic group of acyl carrier protein. Another biosynthetic process that depends upon biotin is the synthesis of glucose in the liver. Pyru-vate, a product of glucose breakdown, is carboxylated to oxaloacetate which is later decarboxylated on its pathway to glucose. Again ATP cleavage is coupled to biosynthesis with the help of biotin.
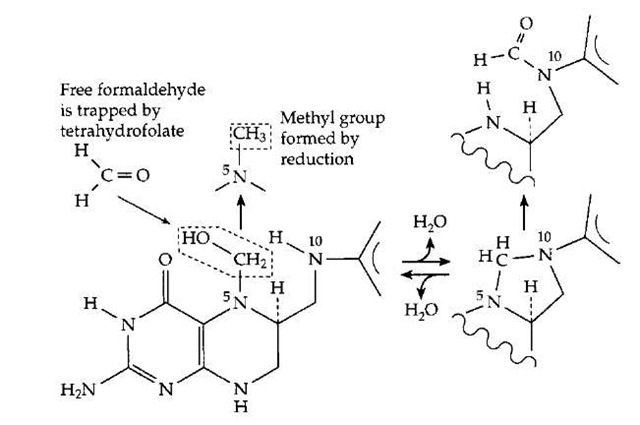
Tetrahydrofolates (THF) interconvert several one-carbon compounds or fragments.As is indicatedin Fig. 18, formaldehyde released from the PLP-dependent cleavage of serine is immediately trapped by THF (Fig. 14). Nitrogen N1 adds to formaldehyde to form a carboxymethyl (—CH2—COOH) derivative which can than react re-versibly with loss of water to form a cyclic adduct (Fig. 18). This compound can be oxidized to the N10-methyl form. Both of these are important intermediates in a variety of biosynthetic processes. The third one-carbon carrier is vitamin B12 which can act as an acceptor, taking the methyl group from methyl-THF to form methylcobalamin (Fig. 7). This compound is transferred to the amino acid homocysteine to form methionine, one of the 20 major amino acids from which proteins are constructed. The reaction accounts for the second human requirement for vitamin Bi2. If the methionine dietary intake is high enough, this reaction is less important, but the enzyme is still essential for remethylation of homocysteine formed when methionine is used in a variety of processes of biological methylation.
The double ring system on which folic acid (Fig. 6) is constructed is known as pterin. In addition to the fo-lates, a number of other pterin coenzymes are found in the human body and elsewhere in nature. Several have shorter side chains at the 6-position on the ring. Some of these compounds are used to color butterfly wings. Another, called biopterin, has a three-carbon side chain that carries two hydroxyl groups. Its reduced form, tetrahy-drobiopterin, is a coenzyme for a series of hydroxylases. Among these is phenylalanine hydroxylase which is lacking in the well-known human genetic defect phenylke-tonuria (PKU). The reduced pterin ring has properties similar to those of FADH2. Molecular oxygen (O2) can add to form a peroxide that can donate an OH group (formally as +OH) to convert phenylalanine to tyrosine. Phenylalanine is toxic to the brain, accounting for the devastating symptoms of PKU. Another pterin derivative is molybdopterin, which has a four-carbon side chain containing two sulfur atoms and an — OH group. The human body, as well as all other organisms, connects this —OH group to a guanine nucleotide to give a complex cofactor somewhat resembling NAD+. The two sulfur atoms, however, bind to an atom of the metal molybdenum. The molybdenum atom is the site at which our bodies oxidize the toxic sulfite (SO3-) to the harmless sulfate (SO4-). This coenzyme, and the associated enzyme sulfite oxidase, are essential to human life. Methane-forming bacteria create a different complex side chain in methanopterin, which replaces tetrahydrofolate in those organisms. Very complex pterin derivatives form the red eye pigments of fruit flies.
FIGURE 18 The functioning of tetrahydrofolates (THF) in oxidation and reduction of single-carbon fragments. A PLP-dependent enzyme cleaves serine (Fig. 14), releasing formaldehyde, which combines in the active center with THF. Formic acid can be converted to formyl-THF. The various THF derivatives supply single-carbon fragments for many biosynthetic processes.
E. Antioxidant Systems
Vitamins C and E, as well as ubiquinones (Fig. 3) and derivatives of the nonmetallic element selenium, together with sulfur-containing proteins, all participate in an elaborate antioxidant system. This system protects us against many of the adverse effects of reduced oxygen compounds such as hydrogen peroxide (H2O2), superoxide (O2-), and hydroxyl (OH) radicals. The system is quite complex and not fully understood. However, ascorbic acid, which can itself form free radicals readily, appears to be a key player. Water-soluble and present in a high concentration, its role seems to be to keep many cellular components reduced. The tocopherols (vitamin E), in their various isomeric forms, scavenge free radicals formed from oxidation of unsaturated fatty acids within cell membranes. Vitamin E is especially effective in removing organic peroxide radicals. Supplementation with dietary vitamin E is being tested for prevention or amelioration of a variety of diseases of aging including atherosclerosis and Parkinson’s and Alzheimer’s diseases. The resulting tocopherol radicals are rereduced by ascorbate in the aqueous phase. Ascorbate can also donate electrons to ubiquinone radicals present in the membranes of the mitochondria. It is within the mitochondria that many damaging radicals are thought to arise as side products of the reduction of O2 that occurs there.
In addition to its antioxidant role, ascorbic acid functions to keep various metallic ions in catalytic centers in their reduced forms. For example, some oxygenases require iron or copper in their Fe2+ or Cu+ states of oxidation. If these protein-bound ions are accidentally left in a more oxidized state they may need to be reduced by ascorbate ions. While this is a protectant role, there are some enzymes for which ascorbate has become a cosubstrate. An example is dopamine j-hydroxylase, which converts dopamine to the neurotransmitter no-radrenaline. The enzyme contains copper which cycles between Cu+ and Cu2+, as it incorporates one atom of oxygen from O2 into its substrate. Ascorbate supplies the electrons for reduction of the second atom of the O2 to H2O. A recent report describes another distinct function for ascorbate ion. It apparently acts as a basic catalytic group for proton abstraction from a water molecule during the action of a glycosyltransferase enzyme, becoming part of the active site of that enzyme.
F. Vitamin A and Vision
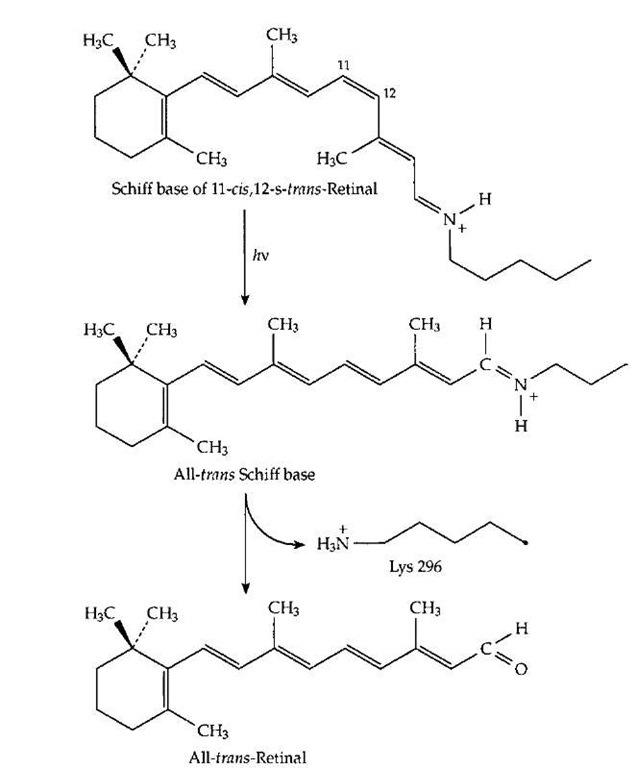
While vitamin A, as retinoic acid, has important hormonal actions (which are not discussed here), its best known function is in vision. Within photoreceptor cells of the retina, and even in certain bacteria, vitamin A aldehyde (retinal,Fig. 1)formsa Schiffbasewith specific lysine side chains of the light receptor proteins. Two of the best known of these receptors are rhodopsin, the pigment present in the rod cells of the mammalian retina, and bacteriorhodopsin, the light receptor of the purple membranes of certain salt-tolerant bacteria. In both of these cases, the protein consists of a similar bundle of seven connected helical segments that pass through a membrane. The retinal Schiff base is inside the bundle, held rigidly in a small "box." In both cases, a particular stereoisomer of retinal is present. In bacteriorhodopsin it is the all-trans isomer pictured in Fig. 1, but in rhodopsin it is the 11-cis isomer shown in Fig. 19. Upon absorption of light, this isomer is converted almost instantaneously into the all-trans form as shown in Fig. 19. The all-trans retinal then leaves the photoreceptor and is replaced with a new molecule of the 11-cis isomer before the photoreceptor can act again. In bacteri-orhodopsin, absorption of light converts the all-trans retinal into the 13-cis isomer within about three trillionths of a second. In both cases, the change in shape of the retinal upon absorption of light induces a small alteration in the geometry and chemical properties of the photoreceptor protein that surrounds the light-absorbing molecule. This is enough to start a chain of signaling events in the retina that leads to a nerve impulse being sent to the brain. In the bacteria, the light absorption is used in a different way to pump a proton from the inside of the cell across the membrane to the outside. The resulting gradient of hydrogen ions (positive charges) across the membrane represents a store of protonic energy similar to that in an electrical condenser. It is used by these cells as a source of energy.
FIGURE 19 The structural change that takes place in the Schiff base of retinal (vitamin A aldehyde) that is formed with specific lysine side chains of the visual pigment proteins upon absorption of a quantum of light. This change triggers a cycle of alterations in the protein that initiates an impulse in the optic nerve.
G. Vitamins A and D as Prohormones
In addition to the coenzyme function of retinal in vision another vitamin A derivative, retinoic acid, is an important hormone with effects on differentiation of cells and tissues. It acts to control transcription of the genetic messages in DNA by binding to specific protein receptors that in turn bind to specific nucleotide sequences of the DNA. The retinoid receptor proteins are a member of the steroid hormone receptor family. Also related to this family are receptors for hydroxylated derivatives of vitamin D.
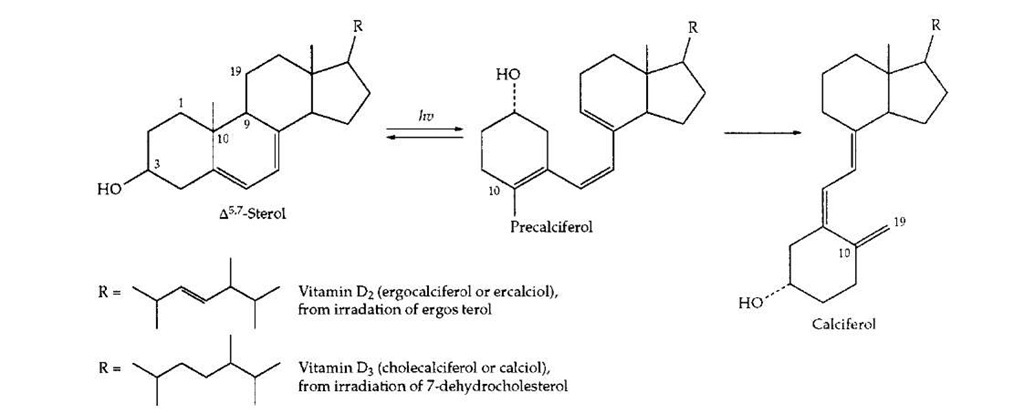
Vitamin D can be viewed as a prohormone which arises by the action of ultraviolet light in the two-step process pictured in Fig. 20. Irradiation of 7-dehydrocholesterol in the skin can provide adequate amounts of vitamin D3 (cholecalciferol or calciol). The closely related vitamin D2 (ergocalciferol) arises from irradiation of the plant sterol ergosterol. This form of the vitamin has been widely used in fortification of milk. However, the natural vitamin D3 is more active in preventing rickets. The term vitamin D1 was dropped when it was found to be a mixture of D2 and D3. The principal function of vitamin D is in the control of calcium metabolism. This control is exerted by polar, hydroxylated compounds of which the most important is 1a,12-dihydroxyvitamin D3 (calcitriol). This hormone is distributed to all parts of the body. In cells of the intestinal lining it promotes uptake of calcium ions. It promotes reabsorption of both calcium and phosphate ions in the kidney tubules and increases blood calcium and depositon of calcium ions in bone.
H. Vitamin K and Blood Clotting
Vitamin K (phylloquinone, Fig. 3), the only form of vitamin K found in plants, functions as an electron carrier in the photosynthetic membranes of the chloroplasts. There it serves to carry electrons from the photosystem I receptor in an electron transport chain related to that of mitochondria. The latter utilizes ubiquinone rather than phylloquinone.
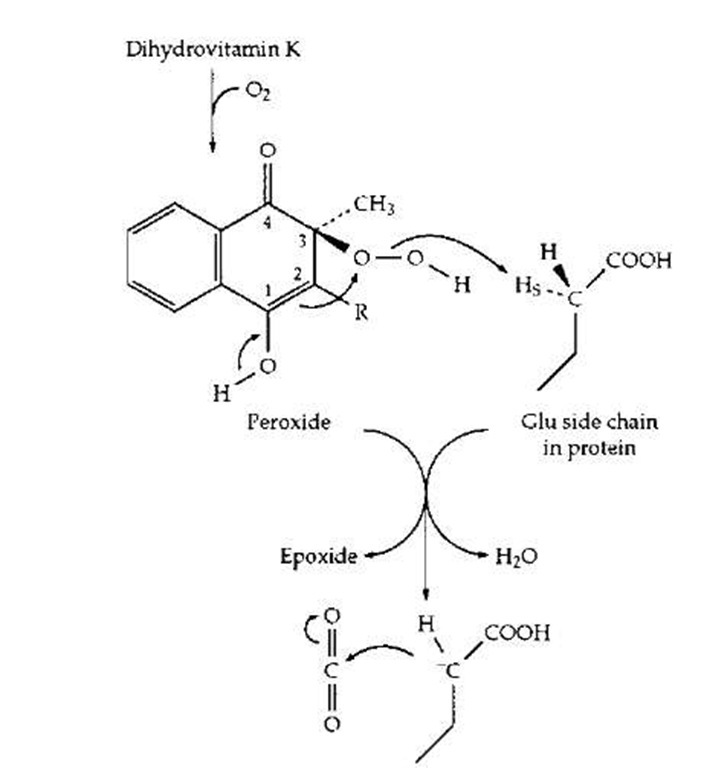
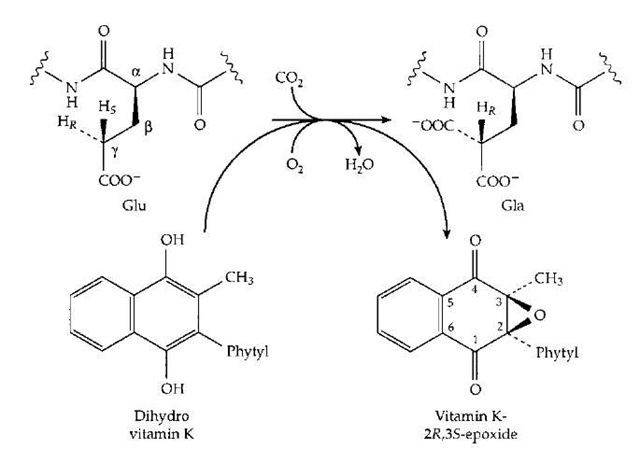
Some photosynthetic bacteria utilize menaquinone, in which the number of isoprenoid units in the polyprenyl side chain is greater. In the human body vitamin K has a quite different and specialized function in the modification of the side chains of glutamic acid units in a small group of proteins. Among these are prothrombin and other blood clotting proteins. Selected glutamic acid side chains (at 10 positions near the N-terminal end of the prothrom-bin chain) are modified by addition of an extra carboxyl group at the gamma (or C4) position of the side chain to give y-carboxyglutamate (Gla) units. The side chain now contains two negatively charged —COO- groups and is able to better bind to calcium ions (Ca2+), which help bind the clotting factors to the phospholipid membrane in the blood clotting complex. Formation of Gla requires both vitamin K and O2, as indicated in Fig. 21. Here the quinone form of vitamin K has been reduced to the dihydro form. It has been shown experimentally to be converted to the epoxide derivative as the glutamyl (Glu) side chain is converted to that of Gla. One possible mechanism is depicted in Fig. 22. The O2 molecule has added to the dihydro-vitamin K to form a peroxide which is used to generate an HO- ion in the active site where it is in a position to remove the hydrogen in the y position to form H2O. The resulting anion adds to CO2 to form the Gla.
I. Recently Discovered Coenzymes and Prosthetic Groups
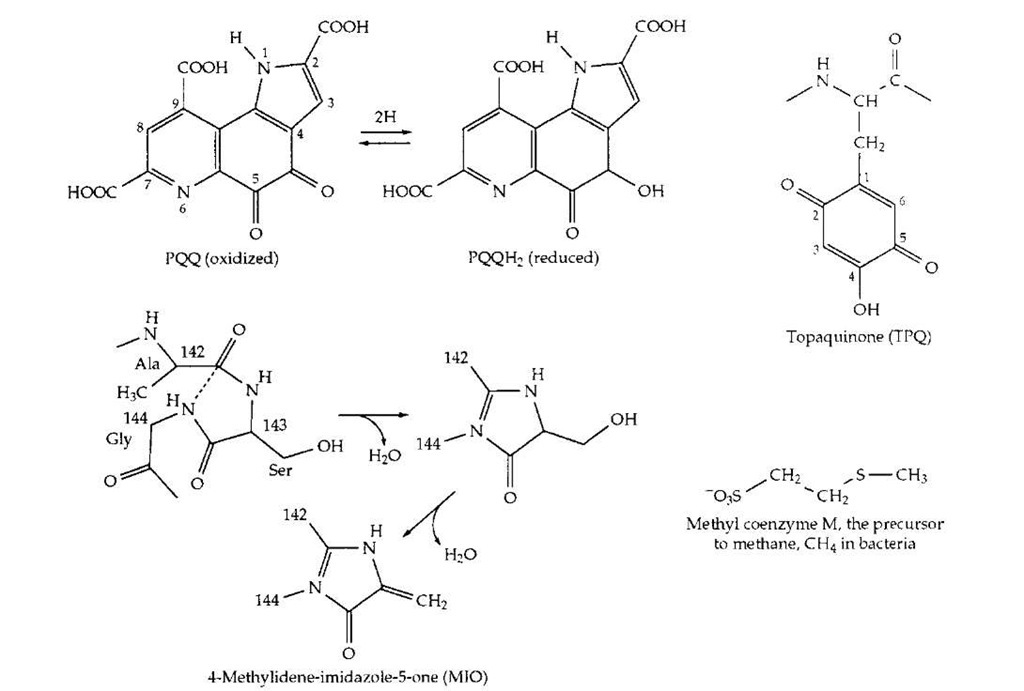
While all of the vitamins may have been discovered, new catalytic cofactors and prosthetic groups are still being found. They are too numerous to mention. A few are shown in Fig. 23. The coenzyme PQQ is a hydrogen carrier that replaces NAD in certain bacterial alcohol dehydrogenases. Topaquinone is a cofactor for amino oxidases found in bacterial, plant, and human tissues. It is a prosthetic group that is formed by oxidation of a specific tyrosine in the active site. It functions together with a nearby copper ion which binds the O2 substrate. The same copper center and O2 are apparently involved in the spontaneous synthesis of the prosthetic group. Formation of topaquinone is only one case of many, in which prosthetic groups are self-assembled using components of the protein that carries the group. Another recently discovered example is formation of4-methylidene-imidazole-5-one(MIO), from glycine and serine units of a protein. The prosthetic group is involved in a previously hard-to-explain isomerization of histidine and phenylalanine. A parallel reaction with phenylalanine initiates the major pathway of synthesis of thousands of aromatic compounds in plants.
FIGURE 20 Creation of the prohormone vitamin D by the action of light on 7-dehydrocholesterol in human skin or on the plant compound ergosterol.
FIGURE 22 A plausible mechanism by which vitamin K acts as a coenzyme to assist in the formation of y-carboxyglutamate side chains in proteins of the blood-clotting system.
FIGURE 21 Scheme showing the coupling of O2-dependent oxidation of vitamin K to its epoxide to the carboxylation of the y -carbon of a glutamyl side chain to a Y-carboxyglutamate (Gla) side chain. One atom of the O2 enters the epoxide while the other enters H2O.
Among specialized coenzymes are a collection of compounds (which include the previously mentioned methanopterin) needed by methane-forming bacteria and compounds such as the light-emitting luciferin of fireflies. Luminous jellyfish make different light-emitting compounds which, like topaquinone and MIO, are made from side chains of amino acids. The list could be continued for pages.
FIGURE 23 Structures of a few recently discovered coenzymes or prosthetic groups.