The major chemical components of cells include the nucleic acids RNA and DNA, polysaccharides (carbohydrates), fatty materials (lipids), and many thousands of different proteins. Proteins catalyze most of the metabolism, the network of chemical reactions by which cells construct their own substance and by which they obtain and utilize energy for all life processes. Proteins, whose structure is dictated by the DNA of the corresponding genes, are precisely constructed. As submicroscopic machines they have moving parts and apparatus for recognizing and binding to other molecules, both large and small. Some coenzymes cooperate with the proteins by carrying electrons, atoms, or molecular fragments. Others help the proteins to catalyze reactions that are difficult or impossible for the reactive groups provided by the amino acid side chains of the proteins.
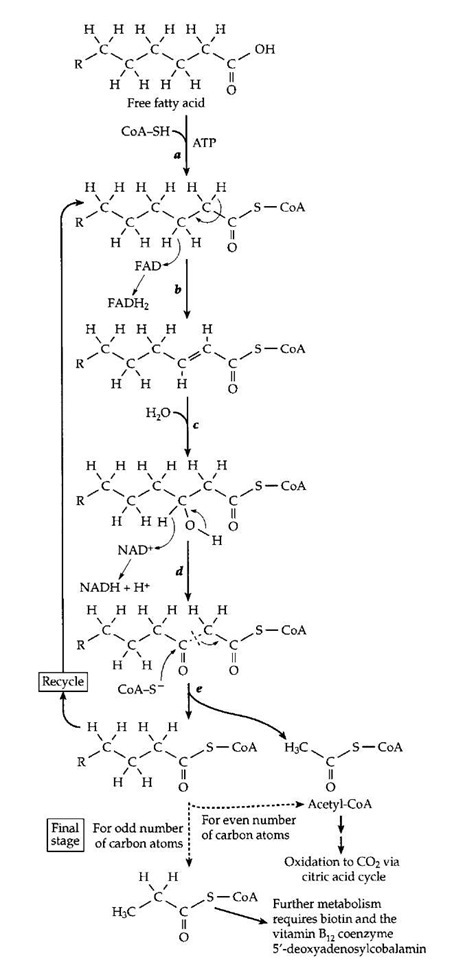
The B-vitamin-containing coenzymes provide a logical starting point for our discussion. As mentioned in Section I, thiamin, nicotinamide, and riboflavin were recognized early in the 20th century as participants in energy metabolism in both animal and yeast cells. Panthothenic acid, biotin, and vitamin B12 were soon added to this list. We now know, in part from complete genome sequences, that all living creatures depend upon these coenzymes to help catalyze a series of central pathways of metabolism. One of these pathways is utilized by aerobic organisms, from bacteria to human beings, for the oxidation of fatty acids (Fig. 12).
A. Coenzyme A, anAcyl Group Carrier and Activator
Coenzyme A, named for its role as an acetyl group carrier, contains the vitamin pantothenic acid as an essential constituent (Fig. 10). The synthesis of this vitamin can be accomplished by green plants, fungi, and most bacteria, but not by the human body. Its unusual chemical structure provides the necessary shape and chemical properties to allow it to bind into crevices within the active sites of a variety of proteins. There it not only fits snugly but has electrostatic bonding interactions that allow the proteins to hold it in just the correct orientation for its function. Curiously, the exact biological need for the unusual structure of the vitamin is still obscure. The chemically functional end of coenzyme A is the sulfhydryl (—SH) group which is added on to the vitamin structure by cells, as shown in Fig. 10. Before coenzyme A can function it must be combined in a thioester linkage with a carboxylic acid such as acetic acid, or a long-chain fatty acid, as illustrated in Fig. 10 (right). It is customary in discussions of metabolism to indicate the bulk of the coenzyme structure as CoA. The free coenzyme is designated CoA—SH, and in a thioester the hydrogen of the — SH group is replaced by an acyl group. The coenzyme has two functions. First, it can carry the acyl group from one protein to the next in a metabolic sequence, such as that of Fig. 12. Second, it can activate a hydrogen atom adjacent to the carbonyl (C=O) group for removal of a proton (H+) by a catalytic group of basic nature, such as —NH2, presentin theprotein. The car-bonyl group in a thioester is an electron accepting group, whose facilitation of the proton removal is often indicated by curved arrows, as shown in Fig. 10 (right). The product of this proton removal is a reactive anion which is able to undergo formation or cleavage of carbon-carbon bonds or dehydrogenation by the riboflavin-containing FAD, as shown in Fig. 12.
FIGURE 12 The use of five different vitamin-containing coenzymes in an important metabolic process, the oxidation of fatty acids (beta oxidation) to carbon dioxide and water.
Other coenzymes and prosthetic groups may also act as acyl group carriers. For the biosynthesis of fatty acids, a shortened version of coenzyme A (phosphopantetheine, Fig. 10), is covalently linked to appropriate proteins. During carbohydrate metabolism, a prosthetic group consisting of bound lipoic acid (Fig. 11) carries acetyl groups. Both acetyl groups and long-chain fatty acyl groups are carried across membranes into and out of mitochondria while attached to the unusual amino acid carnitine (Fig. 4). Carnitine is not a vitamin but acts as a coenzyme.
B. Nicotinamide Adenine Dinucleotide (NAD+) and Flavin Adenine Dinucleotide (FAD), Hydrogen and Electron Carriers
Because of the linkage of the vitamin nicotinamide to the ring of the sugar ribose, NAD+ and its relative NADP+ (which carries an extra phospho group in its structure; Fig. 8) can be reduced by transfer of a hydrogen atom from an alcohol or other suitable substrate to the 4 position of the ring. As illustrated in Fig. 8, the transfer is that of a hydrogen atom plus an electron (a hydride ion H-). NAD+ plays this role in many biological dehydrogena-tion reactions which convert various alcohols into the corresponding carbonyl compounds—aldehydes or ketones. At the same time, many carbonyl compounds are reduced to alcohols. Sometimes the oxidation and reduction processes are linked. A well-known example is the oxidation of glyceraldehyde 3-phosphate during the breakdown of glucose, a process that occurs in bacteria, yeast, and the human body. In all cases NAD+ is reduced to NADH + H+. The latter is reoxidized to NAD+ in the human body, but in lactic acid bacteria the NADH is used (always together with an H+ ion) to reduce pyruvic acid to lactic acid. This provides a balanced fermentation process that requires no oxygen. Under conditions of extreme exertion, e.g., in a 100-meter race, the lactic acid fermentation fuels human muscles. In yeast, a similar fermentation reduces acetalde-hyde to ethanol, indirectly providing energy for the cell.
Why are there two similar coenzymes NAD and NADP? A generalization that holds in many instances is that NAD+ initiates dehydrogenation (oxidation) while NADPH acts as a biological reductant. This permits ox-idative pathways utilizing NAD+ to occur at the same time as reductive processes that utilize NADPH. Cells of aerobic organisms often keep the concentration ratio of the reactants [NAD+]/[NADH] high at the same time that the ratio [NADPH] / [NADP+] is also high. Nicotinamide is a very stable compound, but the coenzyme forms are surprisingly easily destroyed. The reduced forms NADH and NADPH are extremely unstable below pH 7, undergoing ring opening reactions. NAD+ and NADP+ are unstable at high pH, hydroxide ions adding to double bonds in the nicotinamide ring with subsequent destruction of the coenzymes. It is not surprising that our bodies need a daily supply of this vitamin.
Like NAD+, FAD and the simpler riboflavin monophosphate (FMN) often serve as an acceptor of a hydride (H-) ion. However, FAD is a more powerful oxidant than is NAD+. This fact is indicated in a quantitative way by the standard reduction potential, which biochemists tabulate for pH 7 . At this pH the standard hydrogen electrode potential E0′ (for the couple H+/H2) is -0.414 V while that for the powerful oxidant O2 (O2/H2 O) is +0.815 at 25°C. For the NAD+/NADH couple E0′ is -0.32 V and for FAD/FADH2 it is -0.21 V. However, since FAD and FADH2 are often tightly bound as flavoproteins, the value of E0 for flavoproteins varies over a broad range from -0.49 to +0.19 V. The value depends upon the relative strength of binding of the oxidized and reduced forms of FAD to the specific catalytic proteins. In the j oxidation of fatty acids (Fig. 12), the powerful oxidizing properties of FAD make it possible to remove a C3 hydrogen atom as H- either after or concurrently with the removal of a proton from C2. The latter requires participation of a basic group from the protein as well as activation by the CoA thioester group (step b in Fig. 12). The thioester group also facilitates addition of an HO- ion at the C3 position in step c to form an alcohol. The latter is dehydrogenated by NAD+ in step d.
Another important aspect of FAD chemistry is the ability to accept a single hydrogen atom (or a single electron together with a proton) to form a free radical, which we may designate FADff; the dot indicates the reactive unpaired electron. This ability allows FAD or FMN to accept a hydride ion, undergoing a two-electron reduction, then pass the electrons one at a time to an electron-accepting metal center in an electron transport chain such as that found in membranes of the mitochondria. It is at the ends of these electron transport chains that oxygen (O2), brought into the human body through the lungs, combines with four electrons and four protons to form two water molecules. At the other end of the chain — OH groups in a variety of metabolic intermediates are dehydrogenated to carbonyl groups by molecules of NAD+. The resulting NADH transfers its hydrogen (plus a free H+) to FMN within the mitochondrial chain. These reactions, which pass electrons through the electron transport chain, account for most of the oxygen utilized in respiration.
The ability to accept single electrons also allows FAD or FMN attached to some enzyme proteins to react directly with O2, reducing the O2 to hydrogen peroxide, H2O2. The latter has useful functions within cells but may also cause damage. Molecular oxygen (O2) combined chemically with the reduced riboflavin is also used by hydroxy-lases of bacteria and plants to introduce —OH groups into a variety of compounds. A peroxide form of FMN, when bound to the correct protein of luminous bacteria, emits visible light.
Living cells contain many other hydrogen and electron carriers. Among them are lipoic acid (Fig. 11), quinones such as vitamin K, ubiquinone and plastoquinone (Fig. 3), and metal centers containing iron, copper, nickel, manganese, and cobalt.
C. Cleaving C—C Bonds with the Help of Coenzymes
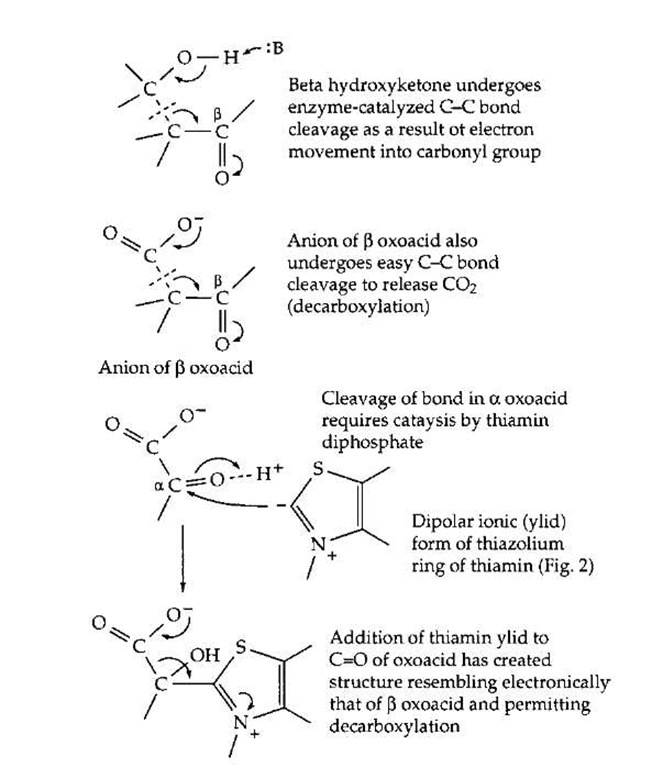
The breakdown of fats, sugars, and other foods as well as the synthesis of body constituents depends upon numerous processes of making and breaking chemical bonds. The cutting and forming of C—C bonds is especially challenging. Enzymes can utilize chemical groupings of an acidic or basic character that are present in the amino acids from which the proteins are made. The acidic —COOH, imidazolium (from histidine), and —NH+ groups serve as proton donors, and the unprotonated forms of these same groups, as proton acceptors. These groups facilitate cleavage and formation of O—H, N—H, and C—H bonds. Certain C—C bonds, e.g., those that are one atom removed from a carbonyl group, can also be broken by proteins using only their own catalytic acid-base groups. This is illustrated in Fig. 13. The carbonyl group provides an electron-accepting center into which electrons can flow temporarily as the C—C bond is broken. Both the cleavage of a j oxoalcohol and a j oxoacid (decarboxylation) are illustrated. In some instances the carboxyl (—COOH) group of a substrate can serve as an electron acceptor. However, the reactivity toward bond cleavage is much higher in a thioester such as that formed from acetyl-coenzyme A (Fig. 10). This high reactivity accounts for one function of coenzyme A. For example, coenzyme A permits the cleavage, by a reverse Claisen condensation, of the fatty acid chain during the j oxidation of fatty acids (Fig. 12, step e). However, not all C—C bonds can be broken using only the chemical groupings of the proteins or of coenzyme A.
FIGURE 13 Activation of C—C bond cleavage by adjacent car-bonyl group (top) and by formation of adduct with thiamin diphos-phate (bottom).
Participation of thiamin diphosphate or pyridoxal phosphate is required for many other C—C bond cleavages. Thiamin diphosphate enables cleavage of an a oxoacid as indicated in Fig. 13. A characteristic of thiamin diphos-phate is that, when bound correctly into an active site, it can lose a proton from its 5-membered thiazolium ring to form the dipolar ionic "ylid" structure shown in Fig. 13. This can add to the carbonyl group of an a oxoacid or an a oxoalcohol to form a covalent compound (adduct) in which the double bond of the thiazolium ring provides the necessary electron-accepting center. The positive change on the nitrogen atom of the ring assists in initiating the chain cleavage. These thiamin-dependent cleavage reactions are found in virtually every major metabolic pathway in higher organisms and in most bacteria. For example, the acetyl-CoA that is generated by j oxidation of fatty acids (Fig. 12) enters the citric acid cycle where the two carbon atoms of the acetyl group are converted to CO2. One essential step in the cycle requires thiamin diphosphate. It is hard to imagine how such metabolic cycles could be organized without thiamin diphosphate.
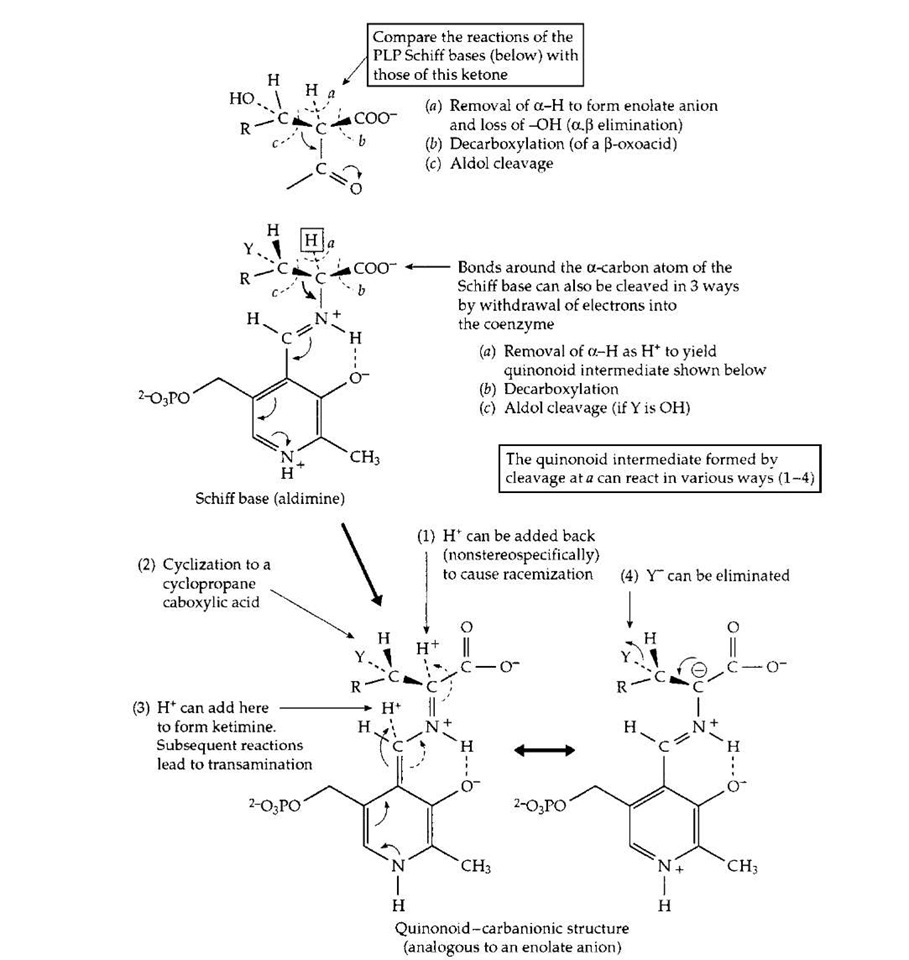
Pyridoxal phosphate, sometimes in collaboration with pyridoxamine phosphate, participates in dozens of different reactions of amino acids, the building blocks of proteins. These reactions involve both the biological synthesis of amino acids and the breakdown of amino acids, e.g., of excess amino acids in the human diet. For these reactions, the PLP is held in place by the enzyme in a location adjoining the binding site for the specific amino acid substrate. In this site an amino group of a protein side chain (a lysine side chain; Protein —NH2) forms a Schiff base linkage in which the carbonyl (C=O) group of PLP is converted to a Schiff base linkage (C=N—Protein) similar to that present in the PLP Schiff base drawn in Fig. 14. This is the "resting form" of the enzyme. Then, in a two-step process, the amino group of the substrate adds to the C=N bond and displaces (eliminates) the Protein —NH2 group to form the substrate Schiff base that is shown in generalized form in Fig. 14. In this Schiff base, one of the three bonds (a, b, c,) may be broken. This is illustrated for cleavage a, removal of a hydrogen atom as H+ by a catalytic group of the protein. The small arrows beside the structure indicate the manner in which the pyridine ring of the coenzyme, with a proton bound to the nitrogen atom of its ring, serves as an electron acceptor in much the same way as does thiamin diphosphate (Fig. 13). The structure resulting from removal of the a-proton of the PLP Schiff base is variously known as a "quinonoid" or "carbanionic" intermediate. Depending upon the specificity of the enzyme in whose site it is formed, this intermediate may react in several ways. In a bacterial racemase a proton may be returned to the a-carbon atom from which it was removed but without stereospecificity, i.e., into either of two positions relative to the other groups surrounding the a-carbon. Some racemases are used by bacteria to convert the stereoisomer known as L-alanine into the less common "unnatural" D-alanine. The latter is incorporated into the bacterial cell wall and helps provide protection to the bacteria against attack by hydrolytic enzymes.
FIGURE 14 The action of pyridoxal phosphate in initiating catalysis of numerous reactions of a-amino acids. Completion of the various reactions requires a large variety of different enzyme proteins.
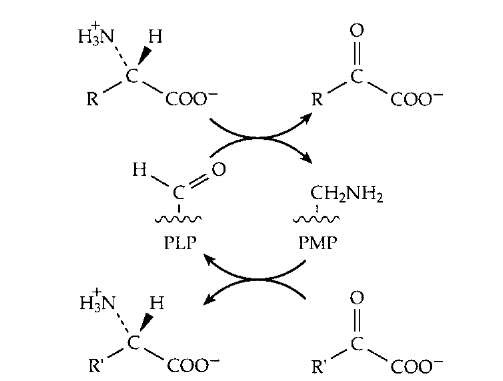
A second mode of reaction of the quinonoid-carban-ionic intermediate is utilized by plants which synthesize an enzyme that acts on the amino acid S-adenosylmethionine to form a cyclic three-membered ring compound aminocy-clopropanecarboxylic acid. This isamajorplanthormone. In a third type of reaction a proton is added back to the coenzyme itself (see Fig. 14) to form what is called a ketimine (not illustrated). This is a Schiff base of pyridox-amine phosphate (PMP, Fig. 5) with an a-oxoacid and is an essential intermediate compound in the important process of transamination (Fig. 14). This process is utilized by all living organisms both in the synthesis of amino acids and in the breakdown of excesses of amino acids. The human body forms several amino acids via transamination. As shown in Fig. 15, this is a reversible sequence involving a cyclic interconversion of PLP and PMP in reaction steps of the type illustrated in Fig. 14.
Yet another reaction for the ketimine illustrated in Fig. 14 is the elimination of a substituent (labeled Y in this drawing) with formation of a double bond. The product of this elimination sometimes decomposes, with loss of nitrogen as ammonia (NH3), but in other cases a molecule carrying a different group may replace Y. Protonation of the new Schiff base that results yields a new amino acid. Several amino acids are made by plants and microorganisms using this reaction sequence. Returning to the top of Fig. 14, notice that cleavage of bond b leads to formation of CO2 and decarboxylation of the substrate amino acid. In this way the amino acid dihydroxyphenylalanine (dopa) is converted to the neurotransmitter dopamine. The latter can then be hydroxylated and methylated to form the hormone adrenaline. Histidine is converted by decarboxy-lation to histamine, a problem compound in allergic reactions, while in the brain, the major excitatory neurotrans-mitter is decarboxylated to gamma-aminobutyrate (gaba). This is the major inhibitory transmitter in the central nervous system and the compound that keeps our brains calm enough to function. Cleavage of bond c (Fig. 14), when R==H and Y=OH (the amino acid is serine) releases the single-carbon compound formaldehyde. This process also requires tetrahydrofolate (Fig. 6). In a converse type of reaction glycine or serine may be condensed with various carbonyl compounds to initiate new biosynthetic pathways. These are often coupled to decarboxylation, which helps to drive the sequence in the biosynthetic direction. One of these yields the red heme pigment of blood.
FIGURE 15 The transamination reaction by which amino groups are transferred from one carbon skeleton (in the form of an a oxoacid) to another to form or to degrade an amino acid.
A third coenzyme that is involved in C—C bond cleavage and formation is the vitamin B12 derivative 5′-deoxyadenosylcobalamin (Fig. 7). In this compound the cobalt-carbon bond is easily cleaved to form a free radical which, in turn, facilitates C—C bond cleavage in the substrate. The details, which are still under study, have been omitted, but Fig. 16 showsa general reaction in which group X is often attached via a C—C bond which is broken. The net result is that a hydrogen atom trades places with group X. These rearrangement reactions, which cannot be catalyzed by proteins alone or by other coenzymes, are quite numerous in various bacteria. However, only one of them occurs in human cells. That is the conversion of methylmalonyl-CoA to succinyl-CoA, the reverse of the succinyl-CoA mutase reaction as drawn in the lower section of Fig. 16. The reaction is essential to the metabolism of propionyl-CoA as is indicated at the bottom of Fig. 12. Propionyl-CoA is carboxylated at the site marked by an arrow in Fig. 17 to form methylmalonyl-CoA. This compound must be isomerized by the vitamin B12-dependent mutase to form succinyl-CoA which can be oxidized to CO2 in the body’s central metabolic pathways. Lack of the mutase is fatal.
FIGURE 16 A family of rearrangement reactions that depend upon free radical formation involving an enzyme-bound form of the vitamin B12 coenzyme 5′ -deoxyadenosylcobalamin (Fig. 7). The rearrangement of (R ) methylmalonyl-CoA to succinyl-CoA (the opposite of the reaction shown here) is one of the two essential vitamin B12-dependent reactions in the human body, and plays an important role in fatty acid oxidation, as is indicated in Fig. 12.