Abstract
Ceramide synthases (CerS) are integral membrane proteins of the endoplasmic reticulum. Six mammalian CerS have been described, with each utilizing fatty acyl CoAs of relatively defined chain lengths for ^-acylation of the sphingoid long chain base. In this topic, we review the main functional features of the CerS proteins, discuss their fatty acid specificity, kinetics, tissue distribution and mode of inhibition, as well as possible posttranslational modifications. We then address the reason that mammals contain six distinct CerS, whereas most other enzymes in the sphingolipid biosynthetic pathway only occur in one or two isoforms. Finally, we discuss the putative roles of CerS and the ceramide derived from the CerS, in signaling pathways and in development of disease.
Introduction
Ceramide (Cer) is an important bioactive lipid that has been implicated in a variety of cell biological processes ranging from regulation of cell growth to cell death and senescence.1-3 The biochemical pathways by which Cer is generated are highly conserved between mammals and yeast.4 Cer is composed of a long chain base (LCB), sphingosine (or sphinganine in the case of dihydroceramide (Fig. 1A)), which is acylated at the free amine nitrogen to form an amide bond. The ^-acylation reaction is catalyzed by ceramide synthases (CerS).
The first molecular characterization of genes involved in de novo Cer synthesis was made about 6-7 years ago with the observation that two genes in yeast, Lag1p and Lac1p, were required for C26-Cer production5,6 (C26 indicates an acyl chain of 26 carbon atoms), the main Cer species found in yeast. Around this time, an earlier version of this topic was published.7 Strikingly, in the earlier version there was no discussion of the molecular identification of CerS; thus, the current topic serves not only to update the earlier topic, but also demonstrates the remarkable progress made in the study of CerS over the past 5-6 years.
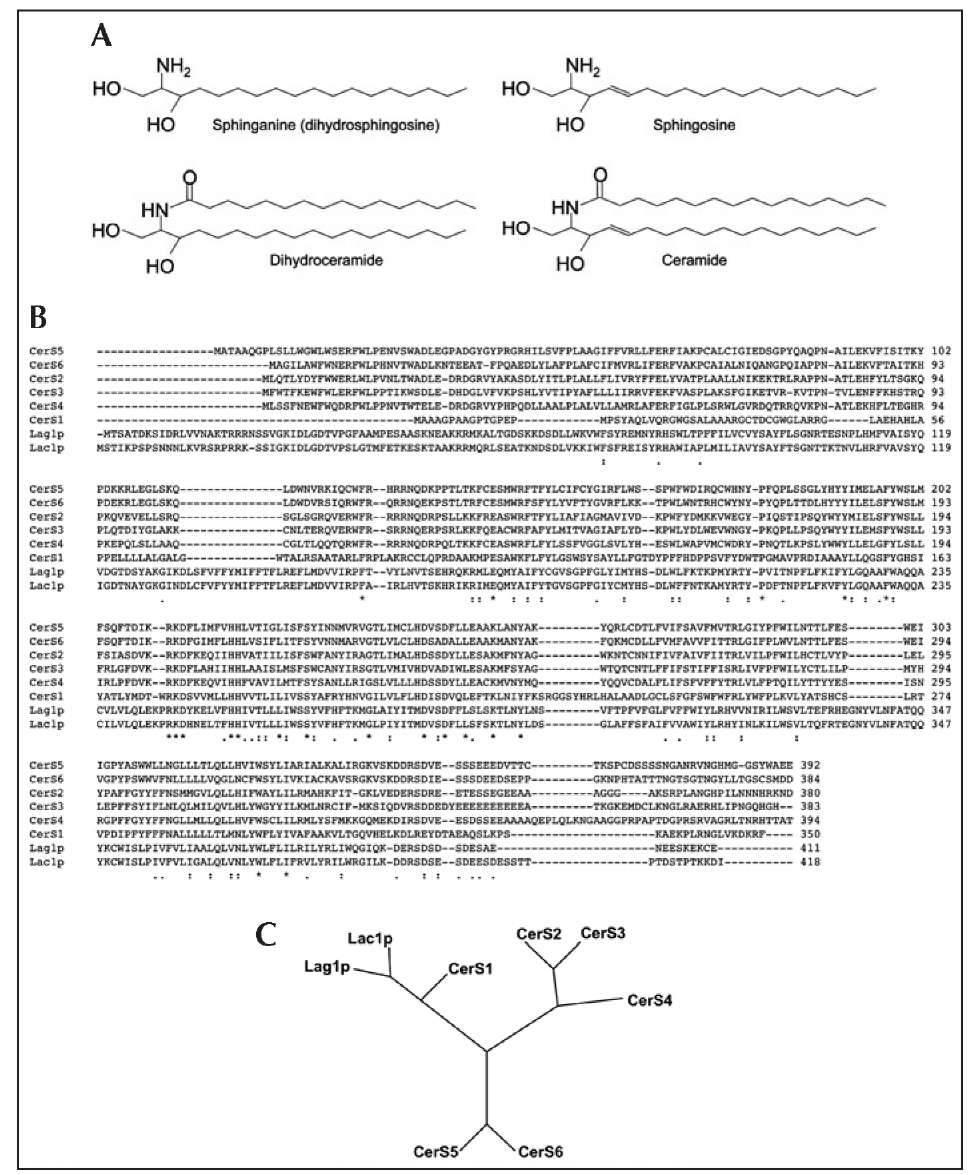
After the discovery of Lag1p and Lac1p, database searches over the next couple of years8,9 revealed six mammalian homologs, which were initially named Lass (Longevity Assurance) genes, but were recently renamed CerS (Ceramide Synthase)10 due to the assignment of their function as genuine ceramide synthases.11 Figure 1B shows the alignment of the sequences of the human CerS family compared to the two yeast proteins. Phylogenetically, CerS1 is more closely related to yeast Lag1p and Lac1p than to the other mammalian homologs (Fig. 1C).10,12 Moreover, all mammalian CerS, except CerS1, contain a Hox-like domain;13 however, this Hox-like domain is unlikely to act as a transcription factor since the first 15 amino acid residues of the Hox domain are missing as are key residues involved in DNA binding. In addition, most of the Hox-like domain can be deleted without affecting catalytic activity.13 Thus, the function of the Hox-like domain in (Hox)CerS (i.e., those CerS which contain a Hox-like domain) is currently unknown.
Figure 1. An overview of CerS biochemistry. A) Structures of CerS substrates and products. B) Alignments (performed using ClustalW2 C) Phylogenetic tree comparing human and yeast CerS.
In yeast, an additional subunit, Lip1, is required for ceramide synthesis,14 but mammalian CerS do not appear to require any accessory proteins for activity.11
The main functional region of the CerS is the TLC (Tram-Lag-CLN8) domain,9,10 a region of ~200 residues also found in other proteins.9 This definition is based on two additional proteins, Tram1 and CLN8; Tram1 was found in a search for homologs of Lag1 in humans. The CerS active site is located in the TLC domain; however, other members of the TLC domain-containing family do not appear to have CerS activity although they may modulate CerS activity.15
Since their discovery, great interest has been shown in characterizing the CerS, with a view to understanding their biochemistry and their biology. It is these aspects that we will now review.
Fatty Acid Specificity, Kinetics and Tissue Distribution
Each mammalian CerS utilizes fatty acyl CoAs of relatively defined chain lengths for Cer synthesis (Table 1), using either sphinganine (derived from the biosynthetic pathway) or sphin-gosine (derived from SL recycling) as the LCB. Thus, CerS1 uses mostly C18-CoA,16 CerS4 uses C18- and C20-CoAs,17 CerS5 and CerS6 use mostly C16-CoA12,17 and CerS3 uses very long chain acyl CoAs (C26 and higher).18 CerS2 can utilize a wider range of fatty acyl CoAs, from C20 to C26, but does not use C16- or C18-CoAs.19 The CerS produce 2-hydroxy (a-hydroxy) Cer with a chain length similar to that of the respective nonhydroxy-Cer.20
A recent study examined the kinetics of Cer formation. Reactions proceeded according to classical Michaelis-Menten kinetics21 and the Km values of all CerS towards sphinganine were in the low ^M range (Table 1). It should be noted that the Km values reported in this study were considerably lower than some reported earlier, in which values as high as 300 ^M were obtained (reviewed in ref. 22). This may be due to the use of detergents in some of these earlier assays, rather than the detergent-free method described in reference 21. Interestingly, CerS4, which can use either C18-CoA or C20-CoA,17has an identical Km value towards sphinganine irrespective of the acyl CoA chain length. This strongly supports the notion that the main biochemical difference between the CerS proteins is in their use of acyl CoAs and that the LCB binding site may be similar between CerS, although this cannot exclude the possibility that different CerS have different affinities towards different LCBs, as appears to be the case with CerS5.17
The CerS are differentially distributed in various tissues, such that subsets of Cer differing in acyl chain length could be made in specific tissues, presumably to meet the different physiological needs of each tissue. Analysis of levels of CerS mRNA in 14 mouse tissues19 demonstrated that CerS2 was the most ubiquitously expressed, with the highest expression in kidney and liver (30-40 molecules RNA/ng total RNA) (Table 1). CerS5 and CerS6 are also expressed in most tissues, but the expression levels (~1-3 molecules RNA/ng total RNA) are much lower than those of CerS2; in another study, CerS5 was found to be highly expressed in mouse lung epithelial cells.23 CerS4 is expressed in most tissues with the skin, leukocytes, heart and liver showing highest expression. CerS3 is exclusively expressed in testes18,19 and skin,19 specifically keratinocytes,20 whereas CerS1 is highly expressed in brain and skeletal muscles.
Table 1. Biochemical and physiological features of CerS
To determine if there is a correlation between CerS mRNA levels and Cer acyl chain lengths in different tissues, the distribution of Cer subspecies was compared to those of the relative expression levels of CerS mRNA.19 The two tissues with the highest CerS2 mRNA levels, kidney and liver, also had the highest proportions of C22 to C24-Cer. Kidney also has high proportions of C22-24 acyl chains in sphingomyelin (SM) and hexosylceramides (HexCer) as does liver, although the N-acyl chain composition of HexCer differs from that of Cer and SM. For the other three tissues examined (brain, testis and skeletal muscle), CerS2 mRNA was less prevalent than the mRNAs of the other CerS and the proportions of C22-, C24- and C24:1-Cer and -SMs are correspondingly lower. Interestingly, for two of these tissues (brain and skeletal muscle), HexCer contains surprisingly high proportions of C22-24-Cer, suggesting that there are factors other than the relative amounts of the CerS mRNA that affect the Cer subspecies distribution, particularly in downstream complex SLs and glycosphingolipids.
Most studies, at least those using immunofluorescence to examine the localization of ectopically-expressed proteins, suggest that CerS are located exclusively to the endoplasmic reticulum with no colocalization with mitochondrial markers;16,17,19 earlier biochemical studies demonstrated that the CerS are found on the cytoplasmic leaflet of the ER.24,25 Nevertheless, there is some evidence, based on biochemical isolation of sub-cellular fractions followed by Western blotting, that CerS can be detected in mitochondria and/or mitochondrial-associated ER mem-branes26,27 (Table 1). Determination of the precise intracellular localization of the CerS awaits the generation of high quality specific antibodies.
Inhibitors
A number of specific inhibitors of CerS have been described, the most notable of which is Fumonisin, a mycotoxin derived from Fusarium. Another fungal-derived inhibitor is Australifungin. A third compound, the immunomodulator, FTY720, was recently shown to inhibit CerS activity.
Fumonisins
Fumonisin (FB) was first shown to inhibit Cer synthesis in 1991,28 before the molecular identification ofthe CerS. Fumonisins are isolated from Fusarium moulds (which occur mainly in maize) and they bear considerable structural similarity to the LCB backbone of SLs. Two derivatives, FB1 and FB2, are potent inhibitors of CerS; FB1 inhibits CerS activity in rat liver microsomes and in isolated hepatocytes with an IC50 value of ~0.1 ^.M.28 Further early studies demonstrated that inhibition occurs via competitive-like inhibition towards both sphinganine and C18-CoA29 and that FB1 and FB2 block the proliferation of LLC-PK1 cells at concentrations between 10 and 35 ^.M; concentrations over 35 ^.M were cytotoxic.30 FB1 is a specific inhibitor of the CerS, as no inhibitory effects were observed on the activities ofother enzymes in the SL biosynthetic pathway.31 In addition, FB1 was shown to block Cer synthesis in cultured hippocampal neurons, which led to significant changes in the rates of neuronal growth.
Since these early studies, FB1 has been extensively used as an inhibitor of the de novo SL biosynthetic pathway in cell cultures as well as in animals and is routinely used to distinguish the effects of Cer generated via the action of sphingomyelinases compared to Cer generated de novo. However, since FB1 inhibits not only Cer synthesis, but also the synthesis of all subsequent down-stream SLs (i.e., SM and glycosphingolipids), care must be taken in the interpretation of results obtained after relatively long times of inhibition with FB1 (i.e., more than one hour). Moreover, upon over-expression of CerS activity in some cultured cells, FB1 elevates Cer levels.8,17 The reason for this is unclear and to date there is no evidence that Cer is elevated by treatment with FB1 in cells that do not overexpress CerS.
Australifungin
Australifungin is a broad-range antifungal agent that acts against human pathogenic fungi at ranges from 0.015 to 1.0 ^g/ml. Australifungin specifically inhibits SL synthesis by blocking CerS activity.34 In crude membranes derived from yeast, the inhibitory concentration of Australifungin was 10 ^.M.35 However, Australifungin is much less widely used as a CerS inhibitor than FB1.
FTY720
FTY720, a sphingosine analog, is in clinical trials as an immunomodulator. The biological effects of FTY720 are believed to occur mainly after its metabolism to FTY720 phosphate (FTY720-P). However, until recently, it was not known if FTY720 itself could interact with and modulate the activity of other enzymes of SL metabolism. Recently, we demonstrated that FTY720 inhibits CerS activity in vitro by noncompetitive inhibition towards acyl CoAs and uncompetitive inhibition towards sphinganine; the EC50 of inhibition varied from ~12-66 ^M depending on the CerS and on the acyl chain length.36 In cultured cells, FTY720 had a more complex effect, with Cer synthesis inhibited at high (500 nM to 5 ^M) but not low (<200 nM) sphinganine concentrations, consistent with FTY720 acting as an uncompetitive inhibitor towards sphinganine. Finally and unexpectedly, elevated levels of Cer, SM and HexCers were observed after short times of incubation with FTY720. These data suggest that some of the effects of FTY720 observed in vivo might need to be re-evaluated in light of its ability to modulate CerS activity.36
Posttranslational Modifications
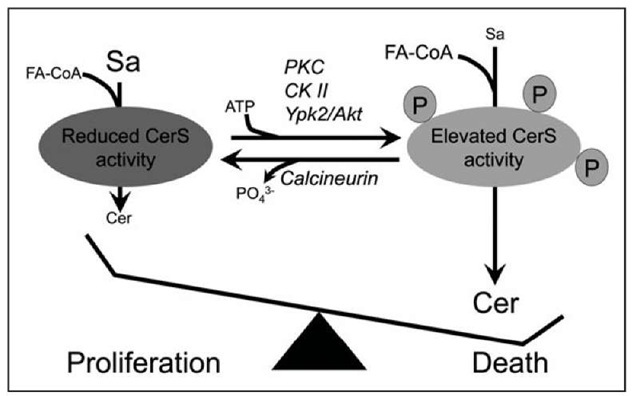
Indirect evidence from over a decade ago, based on rapid changes in CerS activity after various stimuli, suggested that CerS are likely to be modified posttranslationally.37,38 Accumulating evidence supports a role for phosphorylation in the posttranslational modification of CerS. Activation of protein kinase C (PKC) increases de novo Cer synthesis,39 which was attributed to up-regulation of CerS5 activity.40 Deletion of the a’-catalytic subunit of casein kinase II in yeast reduced levels of Cer produced in vitro35 and yeast lacking TOR (Target of Rapamycin) could not synthesize Cer. Ypk2, a kinase activated by TOR2, induces CerS activity and this step is antagonized by the Ca2+/calmodulin-dependent phosphatase, calcineurin.41 Calcineurin has also been shown to negatively regulate the formation of complex SLs42,43 and the overexpression of calcineurin B subunit enhanced the oncogenic potential of HEK 293T cells.44 Thus, indirect evidence suggests that CerS are modulated by phosphorylation, a notion supported by data from high performance mass spectrometry suggesting that mouse liver CerS2 and CerS5 are phos-phorylated.45 Moreover, CerS1 turnover after various drug treatments46 appears to be regulated by the opposing actions of p38 MAP kinase and protein kinase C (PKC); p38 MAP kinase is a positive regulator of turnover, while PKC is a negative regulator of turnover. Pulse-chase labeling experiments demonstrated that CerS1 is phosphorylated in vivo and activation of PKC increases the phosphorylation of the protein.47 The possible role of phosphorylation in regulating CerS activity is illustrated in Figure 2.
Membrane Topology
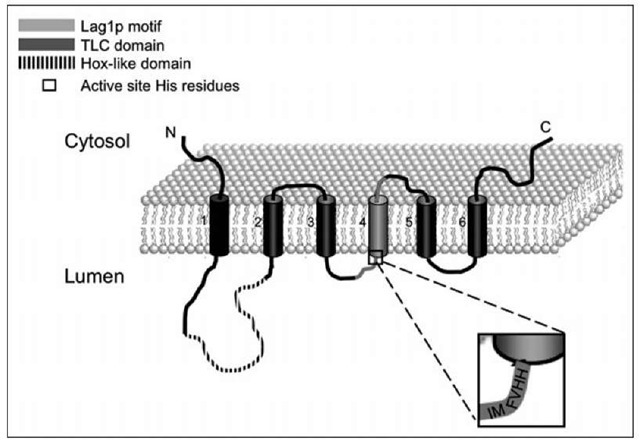
Early proteolytic digestion experiments implied that the active site of CerS faces the cytosol.25 More recently, Igarashi and colleagues suggested that CerS2, 5 and 6 have five transmembrane domains; moreover, the N-terminus of these CerS is inside the lumen of the endoplasmic reticulum and the C-terminus, at least of CerS6, is in the cytosol.12 However, another study suggested that the yeast CerS, Lag1p and Lac1p, have eight putative trans-membrane domains, with both the N- and C-termini facing the cytosol;48 the conserved Lag motif, which contains the potential active site, was suggested to be embedded in the membrane.48 We have tried to resolve this issue using the PHD predicted protein server,49 which suggests that mammalian CerS have six putative trans-membrane domains with both N- and C-termini facing the cytosol (Fig. 3). Verification ofthe trans-membrane topology of the CerS awaits more detailed structural analyses and ultimately, resolution of their crystal structures.
Figure 2. A putative role for CerS in regulating cell death. There have been suggestions that CerS can be phosphorylated (see text). Phosphorylation could occur via PKC (protein kinase C), CK II (casein kinase 2) or Ypk2 (or its mammalian orthologs, SGK and/or Akt/PKB) resulting in activated CerS and generation of pro-apoptotic Cer. Calcineurin could act in the opposite manner, by dephosphorylating CerS. The phosphates shown on the CerS are for illustration purposes only since there are currently no reports on CerS phosphorylation sites.
Figure 3. Predicted membrane topology of CerS.The Hox-like domain (found in CerS2-6), shown as a dashed line, is located in the 1st lumenal loop. The TLC domain, shown in dark gray, begins at the end of the 1st lumenal loop and continues to the end of the 6th transmembrane domain. The Lag1 p motif (which is part of the TLC domain) is shown in light gray and begins in the middle of the 2nd lumenal loop and continues through to the 2nd cytosolic loop. The two conserved histidine residues, which are proposed to be active site residues, are also shown; the enlargement shows the sequence of human CerS5.
Why Are There So Many Mammalian CerS?
The key question related to the physiology of CerS is why there are six distinct enzymes that essentially carry out the same reaction, namely N-acylation ofthe LCB, albeit with strict acyl CoA chain length specificity. A relatively straightforward answer is that ceramides containing specific fatty acids play more vital roles in cell physiology than once thought,10 though what these roles are have not been fully delineated. It is known that ceramides with different acyl chain lengths have distinct biophysical properties50,51 and the ceramides could themselves influence the biophysical properties of the membranes in which they are found, by for instance, differentially interacting with other membrane components. Evidence is also accumulating that ceramides with specific acyl chain lengths are generated in different signaling pathways and that these ceramides can differentially interact with downstream components in such pathways.10,52 Furthermore, the multiple levels ofregulation ofCerS expression17,19,23 and activity37-45 support a vital role for the acyl chain length of ceramide in key events of cell physiology.
Although an integrated picture of how the CerS function together is currently lacking, considerable progress has been made in understanding the roles of individual CerS. CerS1, the first mammalian CerS to be described,16 specifically synthesizes C18-Cer and is mainly expressed in the brain and in skeletal muscle and is almost undetectable in other tissues12,19 (Table 1). CerS1 appears to be involved in cancer regulation and in modulating drug sensitivity. Over expression of CerS1 inhibited the growth of human head and neck squamous cell carcinoma cells (HNSCC) and also increased the chemotherapy-induced apoptosis of these cells.53,54 Moreover, there was a correlation between attenuated C18-Cer levels and the state of clinical disease, suggesting that C18-Cer is an important player in the regulation of HNSCC growth and/or pathogenesis.55 CerS1 also has a unique role in regulating sensitivity to chemotherapeutic drugs;46,56 CerS1 expression led to an increased sensitivity to cisplatin, which is widely used to treat a variety of solid tumors. In response to cisplatin, CerS1 expression increased the activation of the p38 mitogen-activated protein (MAP) kinase and concomitantly CerS1 was translocated from the ER to the Golgi apparatus.46 This data suggests a potential role for CerS1 as a target for improving the efficacy of cisplatin therapy. In summary, CerS1 is regulated by mechanisms that involve PKC, ubiquitination and ER-to-Golgi translocation leading to its eventual proteasomal degradation.47
CerS2, which synthesizes C20-C26-Cer (Table 1) is the most ubiquitously expressed of all the CerS and has the broadest tissue distribution.17,19 In a study on the expression of CerS in the brain, CerS2 was found to have the highest expression of all CerS in oligodendrocytes and Schwann cells and its up-regulation during myelination suggests it is responsible for the synthesis of the majority of SLs in myelin.57 CerS2 is also regulated by a unique mechanism, namely via sphingosine 1-phosphate (S1P). S1P, but not lyso-phosphatidic acid36 interacts with and inhibits CerS2 via two residues that are part of an S1P receptor-like motif, which is found only in CerS2.19 The opposing functions that Cer and S1P play in signaling pathways suggests that this mode of regulation might be of significance in cell physiology and signaling. CerS2 also displays genomic features characteristic of a ‘housekeeping’ gene, although no other CerS genes display these characteristics.19
CerS3 is expressed at high levels in the skin,17 which contains very long acyl chain ceramides that are involved in maintaining the water permeability barrier function58 and in the testes, but is almost undetectable in other tissues.12,19 CerS3 has been postulated to be involved in sperm formation and androgen production;18 indeed, levels of germinal SLs containing very long acyl chains (synthesized by CerS3) increase during postnatal testicular maturation and are important for completion of spermatogenesis.59
CerS4, which uses C18- and C20-CoAs17 is expressed mainly in skin, leukocytes, heart and liver.19 CerS5, which synthesizes C16-Cer, is expressed in most tissues.12,19 C16-Cer is the most abundant short-chain Cer in fibroblasts, endothelial cells and cells of the immune system12,60 and has been shown to be of particular importance in apoptosis.52,61,62 CerS6 is also expressed in most tissues12,19 and produces short acyl chain ceramides (Table 1). However, little is known about the roles of CerS4, 5 and 6 in specific events in cell physiology.
One interesting difference between CerS1 and CerS4 and 5 emerged from a study looking at the role of each of these CerS in mediating drug sensitivity. CerS1 expression rendered cells more sensitive to cisplatin, carboplatin, doxorubicin and vincristine, but in contrast, expression of
CerS4 did not have any effect on the cellular sensitivity to any of the agents tested, while CerS5 expression increased the sensitivity only to doxorubicin and vincristine, but not to cisplatin and carboplatin.46 These results strongly support the idea that the CerS genes are not equivalent in function. Similarly in yeast, the response to stress can vary from one isoform to another. Lac1p, but not Lag1p, is regulated by the pleiotropic drug resistance (Pdr) regulatory pathway, with Lac1p expression ~3 times higher than that of Lag1p.
As summarized in this section, evidence is currently emerging that different CerS play different roles in mediating specific biochemical events and thus specific roles in cell physiology and it is to be hoped that their precise functions will have been clarified by the time of publication of the next edition of this topic.
Roles of CerS in Signal Transduction and Disease
While study of the roles of individual CerS and their modes of regulation is currently in its infancy, much more is known about the roles of Cer and in particular about the roles ofCer containing specific acyl chain lengths. Much of this data has emerged from study of the generation of Cer from SM hydrolysis (via both neutral- and acid-sphingomyelinase), a research area that is somewhat more advanced than study of the generation of ceramides via the biosynthetic pathway.64-66 The cross-talk between the generation of these two pathways is not very well understood; likewise, the coordinate regulation between CerS and other enzymes in the biosynthetic pathway (i.e., serine palmitoyl transferase, glucosylceramide synthase etc), needs to be further studied.
However, a number of studies have shown that Cer generated via CerS can influence cell fate, with up-regulation ofCerS activity causing apoptosis and down-regulation inducing tumor formation. For instance, arsenic trioxide induces the production of cytotoxic levels of Cer by up-regulating de novo synthesis,67 Cer production is stimulated in hypoxia/reoxygenation in NT-2 neuronal precursor cells by the concerted actions of acid sphingomyelinase and CerS5,68 Cer levels are increased via the de novo pathway following p53 up-regulation in leukemia and colon cancer cells and CerS5 transcriptional up-regulation increases C16-Cer levels, ultimately causing cell death.69 A variety of stress stimuli are known to increase Cer levels and some of these act via CerS.70 A list of agents that induce stress is given in Table 2, which also summarizes the putative role of CerS in these processes.
Table 2. Some stress stimuli reported to cause ceramide elevation and apoptosis. For a number of these stimuli, ceramide synthesis via activation of CerS has been implicated, as indicated.
De novo Cer synthesis has also been implicated in a number of diseases,71 such as diabetes,72 cystic fibrosis (CF)73,74 and chronic obstructive pulmonary disease (COPD). In the case of the latter disease, which is characterized by alveolar cell apoptosis, Cer up-regulation via the de novo pathway was shown to be directly involved, since inhibition of de novo synthesis by FB1 and myriocin prevented disease onset.75,76 Cer is also involved in several liver conditions77 such as hepatic ischemia/reperfusion,78 steatohepatitis79 and Wilson disease.80 Cer may also play a role in Alzheimer’s disease,77 with long acyl chain Cer enriched in regions ofthe brain which are vulnerable to Alzheimer’s disease81 with concurrent elevation of CerS2 and CerS4 gene expression.82 Cer is involved in neuronal death83 and white matter dysfunction84,85 and increased Cer levels were suggested to be involved in cerebral ischemia and stroke.77,86 The relationship between Cer generated via de novo synthesis and that generated via sphingomyelinase action is an area of active study in each of these diseases and thus the role of regulation of CerS activity remains to be established.
Conclusion
In this topic, we have summarized the remarkable progress made in study of the CerS genes and proteins since their identification in the early 2000s. Much still remains to be understood, not least whether these genes are coordinately regulated, whether the CerS proteins somehow interact with each other (or with other proteins) and thus modulate their activity and finally, the precise roles of the different Cer species made by each CerS in the various tissues where they are made. The discovery of the CerS has added a new dimension to SL research and the coming years are sure to yield many more unexpected findings.