Mechanisms for Ceramide Generation during Stress
Ceramide does not move spontaneously between cellular membranes and is transported either during normal membrane biogenesis, or with the aid of CERT, a protein that transfers ceramides synthesized at the endoplasmic reticulum to the Golgi apparatus. This insolubility of ceramide suggests that once generated, ceramide is likely to remain localized at the place of its synthesis until metabolized to other sphingolipids. Consequently, the increases in ceramide concentration during stress response are compartmentalized in distinct locations within the cells and might affect diverse sets of down stream targets and responses.
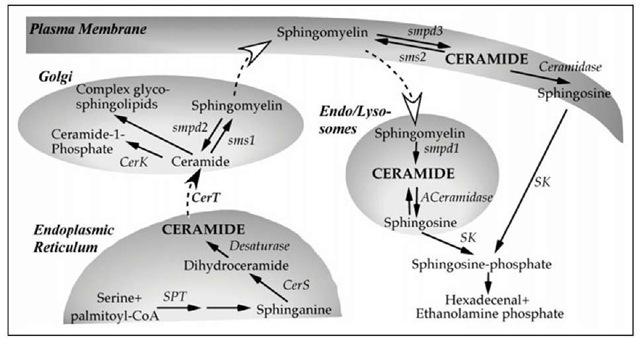
The two main metabolic pathways for generation of excess ceramide during stress response are (i) the de novo synthesis in the endoplasmic reticulum and (ii) the turnover of sphingomyelin (SM) either at the plasma membrane or in the endosomal/lysosomal compartment (Fig. 2). A number of agonists have been shown to activate these pathways leading to transient elevation in ceramide. The magnitude and temporal pattern of ceramide accumulation is further influenced by the activity of ceramidases, SM synthases, ceramide kinase and glucosyl/galactosyl ceramide synthases. These enzymes catalyze the conversion of ceramide to other sphingolipids and some agonists seem to coordinately regulate both, the ceramide production and turnover.20,96
Role of the De Novo Pathwayfor Ceramide Generation in Cellular Stress Response
The de novo pathway for synthesis of ceramide consists of 4 reactions: the serine palmitoyltransferase (SPT), which condenses palmitoyl-CoA and serine into 3-ketosphinganine, the 3-ketosphinganine reductase that generates sphinganine, the (dihydro)ceramide synthase, which acylates sphinganine to dihydroceramide and the dihydroceramide desaturase, which converts relatively inactive dihydroceramides to ceramides. Stimulation of the de novo pathway during cellular stress response happens through up-regulation of the activity of SPT and/or (dihydro) ceramide synthase.
Figure 2. Metabolic pathways responsible for ceramide synthesis and degradation. The pools of ceramide involved in cellular response to stress are depicted in capital letters. Name of the enzymes are in plain italic. The names of relevant subcellular organelles are shown in bold italic. Black solid arrows are used to depict metabolic conversions. Black dashed arrow indicates protein-mediated transfer. White dashed arrow indicates vesicular transport. Abbreviations: SPT: Serine Palmitoyltransferase; Cers: Ceramide synthases; CerT: ceramide transfer protein. SMS1 & 2: sphingomyelin synthase 1 & 2; smpd1: Acid Sphingomyelinase, smpd2: Neutral sphingomyelinase1, smpd3: Neutral sphingomyelinase 2. CerK1, Ceramide Kinase1; SK: sphingosine Kinase. ACeramidase: Acid Ceramidase.
The enzymes of the de novo synthesis of ceramide are in the endoplasmic reticulum. The newly generated ceramide is actively transported to the Golgi apparatus, where it serves as a rate-limiting substrate in the synthesis of complex sphingolipids, like SM and gly-cosphingolipids. It is noteworthy that some of the known ceramidases may also act as ceramide synthases in a CoA-dependent and independent manner as they exhibit reverse activity. This alternative route for ceramide synthesis is often referred to as the salvage pathway and its role in stress response is not well understood.
The activation of the de novo pathway apparently leads not only to an elevation in ceramide but also to increases in the concentration of SM and glycosphingolipids. Such parallel accumulation is documented in response to LPS, palmitate treatment and heat shock, among others and implies that a wide range of cell functions might be affected through specific and nonspecific mechanisms. Inhibitors of glucosylceramide synthase and labeling with radioactive precursors have been successfully used to elucidate the specific role of ceramide in each case.
De novo synthesis of ceramide is target of a number of fungal inhibitors like fumonisin B1, which inhibits dihydroceramide synthase and the reverse reaction of some ceramidases97 and myriocin/ISP-1,98 cycloserine99 and beta-chloroalanine,100 all of which inhibit SPT. These widely used inhibitors remain a critical test of establishing whether the de novo pathway is involved in a particular cellular response (reviewed in Merrill101 and in Perry96).
The activation of the de novo pathway during conditions of stress was initially discovered in yeast, where it regulates growth and the response to heat or osmotic stress (reviewed in Dickson102 and in Meier et al103). However, in yeast the signaling mediator is not ceramide but the free long chain bases, phytosphingosine and dihydrosphingosine, most likely reflecting the specifics of sphingolipid synthesis in yeast. Later, the activation of the de novo pathway in C. Elegans in response to ionizing radiation was described and found to be involved in activation of the CED-3 caspase104 and the resulting apoptosis of germ cells.
In mammalian systems, the de novo pathway seems to play a prominent role in cellular response to heat or chemical stress,105 septic shock,106 lipo-apoptosis107 and insulin resistance,108 as well as in receptor-dependent and -independent induction of apoptosis by variety of chemotherapeutic agents like etoposides109 and doxorubicin.110
Heat Stress
In the human acute lymphoblastic leukemia cell line Molt-4, heat shock induces more than twofold increase in total ceramide levels with C16 ceramide being the major species affected. This accumulation of ceramide has been linked to the induction of c-jun and apoptosis.105 Labeling with tritiated palmitate has shown an accumulation of ceramides, but not sphingoid bases, thus confirming that in contrast to yeast, where sphingoid bases mediate heat shock, in mammals, ceramide is apparently involved. Both, myriocin and fumonisin B1 inhibit the increase in total ceramide mass thus confirming that ceramides produced upon heat shock were products of SPT and ceramide synthase activity.
Septic Shock
Similar observations delineate a role of the de novo synthesized ceramide in septic shock in vitro as well as in vivo.106 Administration of LPS or cytokines to rabbits increases hepatic sphingolipid biosynthesis leading to the accumulation of ceramide, SM and glycosphingolipids. Studies in cell lines link the stimulation of the de novo pathway to the activation of the MAP kinases and NFkB and respectively to the innate immune response. In nonhepatic tissues the elevation in ceramide biosynthesis during septic shock is correlated with the elevated glycosphingolipid synthesis that seems to play a role in pathogen recognition.
Activation of ceramide synthesis in the liver has been linked to production and secretion of lipoproteins enriched in ceramides, sphingomyelins and glycosphingolipids. The functions of these lipoproteins with "altered" sphingolipid content was not well understood; it became clear however that the activation of the de novo synthesis during septic shock is paralleled by activation of another ceramide-generating enzyme, the Zn2+-dependent secretory form of ASMase, termed SSMase. This enzyme hydrolyses SM in the Low Density Lipoproteins (LDL), leading to a robust increase in LDL ceramide content. Several lines of evidence suggest that LDL that are rich in ceramide might mediate injury to the arterial wall during inflammation. LDL extracted from atherosclerotic plaques contain higher ceramide content as compared to LDL isolated from the plasma.111 Experimentally-induced elevation in LDL ceramide content has been further linked to higher rate of LDL aggregation112 and oxidation, as well as with an increased potential to induce apoptosis in microvascular endothelial cell.113
Lipotoxicity and Insulin Desensitization
Consumption of diet rich in saturated fats (also known as Western diet) is the main risk factor for the development of insulin resistance, hyperglycemia and atherosclerosis. Palmitic acid is the main component of the Western diet and is linked to excessive accumulation of lipids in lean tissues, mainly muscle and liver, lipotoxicity and insulin resistance. Because SPT has almost exclusive preference for the CoA-thiol ester of palmitate, numerous studies have investigated whether Western diet affects de novo synthesis of ceramide. These studies have shown that excess palmitate (delivered via the consumption of Western diet, i.v. infusion, or directly added to cells in culture) stimulates the flux through the de novo pathway resulting in accumulation of ceramide, SM and glycosphingolipids.58,114 Inhibition of SPT by myriocin prevents not only the palmitate flux through the pathway but also inhibits lipotoxicity, improves insulin response and leads to better glucose regulation.
The exact mechanism by which ceramide affects insulin response is not completely understood. Schmitz-Peiffer et al115 observed that the accumulation of ceramide in myotubes exposed to palmitate was paralleled by inhibition of Akt/PKB. Cotreatment with myriocin, cycloserine, or fumonisin B1 restored insulin-stimulated Akt phosphorylation, even in the presence of excess palmitate, suggesting that palmitate-induced stimulation of de novo synthesis is required for inhibition of insulin responsiveness. Recent data however imply that in vivo, the diet-induced ceramide increases have to be accompanied by increased triacylglycerides synthesis and accumulation in order to affect the overall insulin response. Furthermore, stimulation of the de novo synthesis of sphingolipids and that of triacylglycerides seems to be correlated in liver.59
Programmed Cell Death
Perhaps, the most extensively studied cellular response to ceramide is the induction of programmed cell death or apoptosis. Activation of(dihydro)ceramide synthase in particular is implicated in endothelial cell death induced by TNFa,116 in daunorubicin,110 doxorubicin and gemcitabine-in-duced apoptosis and may account for some aspects of the toxicity of phorbol esters,117 angiotensin II,118 cannabinoids119 and ischemia-reperfusion. In the latter case, investigation of intracellular sites of ceramide accumulation reveals that the elevation of ceramide is in mitochondria and is caused by the activation of a mitochondrial ceramide synthase via posttranslational mechanisms. Furthermore, ceramide accumulation appears to cause mitochondrial respiratory chain damage that could be mimicked by exogenously added natural ceramide to mitochondria.12
The recent cloning and characterization of6 members of the ceramide synthase family CerS1-6 (also known as longevity assurance gene 1-6 (LASS1-6)) provided the opportunity to finally begin studying the role of these enzymes in apoptosis in more details. CerS1 but not CerS2-6 for example, was found to be sufficient and required for apoptosis in response to gemcitabine/ doxorubicin treatment.120 Notably, the gemcitabine/doxorubicin combination treatment was discovered to increase only the levels of C18-ceramide, the preferable substrate for CerS1. These and other studies have provided experimental evidence to the earlier assumptions that not only the type of sphingoid base (i.e., sphingosine vs sphinganine) but also the fatty acid length and degree of saturation influences the biological effectiveness of ceramide.
The role of the de novo pathway in apoptosis had also been investigated in different animal models. Inhibition of the enzymes controlling the de novo pathway prevents alveolar cell apoptosis, oxidative stress and emphysema (the prevalent disease caused by cigarette smoking) in both rats and mice,121 delays the progression of atherosclerotic plaques in mice122 and ameliorates some of the pathological consequences of spinal cord injury.123
Autophagy
The final paradigm to be discussed is the role of the de novo synthesis of ceramide in autophagy, an evolutionary conserved cytoprotective mechanism that sustains cells during periods of nutrient limitation, but under certain conditions may lead to mammalian cell death. Ceramide addition is sufficient to induce autophagy in some cells and a correlation between the rate of autophagy and ceramide synthesis has been observed in same pathological conditions suggesting a mechanistic link between ceramide, autophagy and disease (reviewed in Zheng et al2). Activation of de novo ceramide synthesis mediates autophagy in response to a bioenergetic crisis resulting in the rapid and profound down regulation of nutrient transporter proteins.124 Ceramide is also shown to mediate the tamoxifen-dependent accumulation of autophagic vacuoles in the human breast cancer MCF-7 cells and to counteract interleukin 13-dependent inhibition of macroautophagy in HT29 cells.125 The mechanism seems to involve the pro-autophagic protein beclin 1.
Mechanisms of Activation of De Novo Synthesis of Ceramide during Stress
Regulation of SPT at a transcriptional level has been seen with a number of agents, including endotoxin and cytokines,106 UVB irradiation126 and others.127 Induction of both form of SPT, SPT1 and SPT2, occurs in balloon-injured rat carotid artery.128 In contrast, long-term consumption of food rich in palmitate is correlated with increases in SPT1 protein but not mRNA level.59 Activation of SPT occurs also posttranslationally in response to etoposide33 and to heat shock in yeast.34 Mitochondrial injury in cerebral ischemia/reperfusion activates ceramide synthases via posttranslational mechanism which is dependent of JNK.12 Cers1 mRNA transcription is up-regulated in response to a gemcitabine/doxorubicin combination treatment,120 while cisplatin is shown to cause a specific translocation of Cers1 from the endoplasmic reticulum to the Golgi apparatus.129 The de novo pathway is also modulated through a negative feedback mechanism determined by the rate of sphingolipid degradation in the lysosomes since lipoproteins, sphingosine phosphate130 and ASMase activity59 seems to inhibit the flux through it. This might be a mechanism to prevent excessive synthesis of ceramide during normal healthy state of the cell.
Role of the Sphingomyelinases in Cellular Stress Response
The SMase family is a group of biochemically and genetically different enzymes all of which hydrolyze SM to ceramide and phosphorylcholine. SMase activities with neutral and acidic pH optima are found in most mammalian cells and an enzyme active in alkaline pH is localized in the intestinal wall. Currently, research is focused on 4 genes encoding different mammalian SMases: smpdl encodes two forms of acidic SMase, one associated with the endosomal/lysosomal compartment (ASMase) and a second one found in the plasma and the conditioned medium of stimulated cells (SSMase). smpd2 and smpd3 encode the Neutral SMase 1 (nSMase1) and 2 (nSMase2), both of which are Mg2+-dependent but differ in their subcellular localization and role in signaling. Data from several labs have shown that in mammalian cells, nSMase1 is a housekeeping enzyme with no particular function in signaling. The recently cloned zebra fish nSMase1, however seems to mediate heat-induced apoptosis in zebra fish embryonic cells.131 In mammalian cells, nSMase2 is regulated by cytokines like IL-1P and TNFa and mediates some of the cytokine effects.132-134 The recently cloned smpd4, is suggested to encode a novel form of NSMase, nSMase3 that is found predominantly in skeletal muscle and heart.
Neutral Sphingomyelinase
Hepatic Acute Phase Response
The acute phase response of liver is an essential component of the systemic host response to bacterial infection and injury and requires IL-1P, a prototypic inflammatory cytokine. Activation of NSMase by IL-1 P resulting in transient elevation in ceramide concentration is observed in number of cells including hepatocytes, mesangial cells, EL-4 cells and it has been related to activation of TAK-1, JNK and NF-kB, all of which have important roles in the IL-1P cascade.135-137
In hepatocytes, specific silencing of nSMase2 with siRNA has only a minimal effect on the basal cellular NSMase activity, however it results in a complete inhibition of the IL-1 P-stimulated NSMase activity.138 Therefore, nSMase2 is probably an inducible enzyme that contributes little to the basal turnover of SM, but at the same time it is also the only neutral SMase activated by IL-1 P. The role of NSMase2 in the IL-1 P signaling cascade is rather complex: seemingly, the activation of nSMase2 modulates the pattern of phosphorylation of JNK by IL-1P and respectively the magnitude of transcriptional induction of the hepatic acute phase proteins like C-Reactive Protein, a1 Acid Glycoprotein and Insulin-like Growth Factor Binding Protein-1. nSMase2 activation in hepatocytes leads to potentiation of JNK phosphorylation due to stabilization of the IL-1P receptor-associated kinase-1. The latter most likely involves a phosphatase, like the ceramide-activated protein phosphatase 2A (PP2A).139
Vascular Inflammation
Activation of nSMase2 by another pro-inflammatory cytokine, TNFa is well documented in cells of the vasculature and various cancer cells.132,140,141 TNFa-induced nSMase2 activation is a prerequisite for endothelial nitric oxide synthase activation in HUVEC cells,134 as well as for the up-regulation in A549 lung epithelial cells of vascular cell adhesion molecule and intracellular adhesion molecule 1,141 all of which have prominent roles in vascular inflammatory responses.
Apoptosis
Studies into the activation of NSMase during apoptosis have focused mainly on the role of NSMase-mediated ceramide production in the apoptosis induced by 55 kDa receptor for TNFa.142 A protein factor associated with NSMase activation, FAN, which interacts with the membrane-proximal domain of the p55 receptor and couples stimulation of the receptor to neutral SMase activation, is required for TNFa-induced ceramide generation, caspase processing and apoptosis.143 Subsequent work in MCF-7 cells found that activation ofNSMase in TNFa-stimulated cell death is upstream of mitochondrial changes, cytochrome C release and caspase-9 activation.144
Oxidative stress-induced cell death may also be attributed to activation of NSMase. Free oxygen (H2O2), but not nitrogen (ONOO~) radicals specifically activate nSMase2, while silencing nSMase2 prevents H2O2-induced apoptosis, but had no effect on ONOO~-induced apoptosis.83
Very recent studies had began to suggest that a novel form of neutral SMase, nSMase3 is a DNA damage and nongenotoxic stress-regulated gene that is deregulated in human malignancies and modulate the sensitivity of cancer cells to adriamycin-induced cell killing.145 Early studies seem to suggest that at least the overexpressed nSMase3 can be activated by TNFa within seconds of stimulation.
Growth Arrest
A number of studies have established a role for NSMase-generated ceramide in regulating the cell cycle and mediating growth arrest, possibly through dephosphorylation of retinoblastoma protein and/or regulation of cyclin dependent kinases. Serum withdrawal causes activation of NSMase and cell cycle arrest at G0/G1 in Molt-4 cells.146 Interestingly, a study focusing on genes up-regulated during confluence-induced arrest had initially identified nSMase2 as a confluence-arrest gene, CCA1, in rat 3Y1 cells.147 In line with that, nSMase2 seems to mediate confluence-induced growth arrest of MCF-7 cells. The latter was preceded by confluence-induced translocation of nSMase2 to the plasma membrane.148
Aging and Cancer
Constitutive up-regulation of nSMase2 and elevation of ceramide concentration during aging has been observed in liver, brain, macrophages and other tissues.149 This aging-associated elevation in ceramide seems important for the onset of aging process since it has been causatively linked to hyperresponsiveness to IL-1 P70 and LPS41 and to deregulation of nitric oxide production in endothelium.71 A substantial decline in hepatic GSH content, which is characteristic for the aging process is responsible for this constitutive increase in nSMase2 activity.150 nSMase2 apparently follows a pattern of regulation consistent with "developmental-aging" continuum, since in animal models of delayed aging, like calorie-restricted animals, the aging-associated changes in NSMase activity and function are reversed.150 In cellular model of senescence, similar induction of NSMase activity and accumulation of ceramide has been observed in senescent cells and linked to the decline in proliferative capacity and onset of senescence.72
In contrast, a somatic homozygous deletion specifically targeting nSMase2 (Smpd3) is found in a genomic screen for gene copy losses contributing to tumorigenesis in a mouse osteosarcoma model, while loss-of-function mutations in smpd3 gene were identified in 5% of acute myeloid leukemia and 6% ofacute lymphoid leukemia cancers. It has been suggested that the mutation could be linked to a defect in plasma membrane translocation of nSMase2 and decreased responsiveness to TNFa. Reconstitution of smpd3 expression in mouse tumor cells lacking the endogenous gene enhances the TNFa-induced reduction of cell viability.151
Mechanisms of Activation of NSMase
(i) Translocation; Studies have shown that nSMase2 can be translocated to the plasma membrane when cell confluence is reached.132 This translocation of nSMase2 is also required for the confluence-induced cell cycle arrest to ensue.152 In oligodendroma-derived cells, a regulated translocation of NSMase2 to the caveolae, which are the signaling domains of the plasma membrane153 has been observed, while studies with highly differentiated nonproliferating primary hepatocytes have shown that the overexpressed nSMase2 is localized constitutively at the plasma membrane.133 These data suggest that translocation of nSMase2 to the plasma membrane might be important mechanisms for regulation of its activity in situ by bringing the enzyme into contact with its substrate. Pharmacological inhibitors and specific siRNA has implicated the novel PKC, specifically PKCS, in TNF and PMA-stimulated nSMase2 translocation to the plasma membrane.
(ii) NSMase as a redox-sensitive enzyme. The major scavenger of reactive oxygen species, GSH, has been found to be a reversible inhibitor of cellular NSMase activity.33,154 This is noteworthy, because depletion of cellular GSH content is observed in conditions of increased oxidative stress. The modulation of NSMase activity by GSH was first established in the context of regulation of TNFa signaling and apoptosis.33,154 Later, the ability of GSH to affect the sensitivity of T47D/H3 breast cancer cells to doxorubicin was attributed to the inhibitory effect GSH has on NSMase activity.155 A correlation between oxidative stress and NSMase activity was also found in long-lived rats on vitamin Q10 enriched diet156 and in astrocytes treated with vitamin E.157 Finally, recent research has shown that specific downregulation of nSMase2 with siRNA blocks H2O2-induced apoptosis of human aortic endothelial cells, identifying nSMase2 as a redox-sensitive protein.158 Detailed analyses of sensitivity of nSMase2 to GSH in hepatocytes suggest that GSH depletion exerts its effect on NSMase activity following a sigmoid dose dependent curve. A rapid and significant activation of NSMase is observed only when hepatic GSH concentration drops below 30% of its basal level, implying that there is a threshold required for NSMase activation during oxidative stress.150 Interestingly, nSMase1 is also sensitive to changes in the GSH/GSSG ratio.159 This suggests that redox sensitivity might be a common property of neutral SMases.
Acid Sphingomyelinase
Endotoxic Shock
Patients with severe sepsis exhibit an enhanced SMase activity in plasma. The increase is correlated with the severity of illness and the fatal outcome.160 Studies in humans and mice administered with LPS confirm these observations.39 Deletion of ASMase protects against LPS-induced elevation in the plasma SMase activity161 and attenuates endothelial apoptosis and animal death43 implicating a secretory isoform ofASMase (SSMase) in apoptosis and organ failure in sepsis. The physiological and biochemical properties of this SSMase are not well understood. It has been postulated that SSMase degrades SM in the secreting cell outer membrane leaflet, which contains almost % of the cellular SM. This SM pool is seemingly inaccessible for other cellular SMases, since the active center of nSMase2, the sole plasma membrane-associated SMase, is facing the cytosolic leaflet of the membrane. These proposed autocrine functions of SSMase however had not been rigorously tested. In turn, substantial evidence had implicated SSMase in modifying the SM/ ceramide content of circulating LDL as discussed earlier in the topic.
Apoptosis (reviewed in ref. 162)
Irradiation of tumor cells with ionizing radiation52,163-165 transiently activates ASMase with maximal activity detected between 1 to 10 min post irradiation. The activation of ASMase has been linked to the ability of radiation to induce apoptosis since ASMase deficiency causes apoptosis resistance in various tissues in ex vivo or in vivo experiments.163-165 Genetic restoration of the activity also restores the radiation effects indicating that ASMase mediates apoptosis via ceramide, at least in some cells including B cells, endothelial cells or mesothelium, lung epithelial cells, MCF-7 breast cancer cells, etc. However, some other cell types, for example, thymocytes, remained sensitive to radiation in ASMase-deficient mice suggesting that radiation effects are mediated by different mechanisms depending on the cell type.
ASMase is also involved in death receptor-mediated apoptosis. Stimulation via the CD95 receptor leads to ASMase activation and ceramide accumulation that precedes the induction of cell death.166-168 Furthermore, studies with fibroblasts from Niemann-Pick disease type A patients, who exhibit deficiency of ASMase and hepatocytes from ASMase null mice show that ASMase activation is required and sufficient for CD95 induced apoptosis.168,169
Viral and Bacterial Infections
Cellular A SMase activity seems to play an important role in susceptibility of mammalian organisms to microbial infections. ASMase-deficient mice were found to be more sensitive to infections with the Gram-negative bacteria L. monocytogenes, due to a defect in lyso-phagosomal fusion. In wild-type macrophages, the phagosome rapidly fuse with the lysosomes to form a phago-lysosome and to kill and digest bacteria, while in macrophages deficient in ASMase the process is slower and leads to inefficient transfer of lysosomal antibacterial hydrolyses into phagosomes.170 Apparently, ASMase is required for the proper fusion of late phagosomes with lysosomes.171 ASMase-deficient mice were also more susceptible to infection with Sindbis virus, an enveloped virus with a single-stranded RNA that is involved in fatal alpha virus encephalomyelitis, due to more rapid replication and spread of the virus in the nervous system.172
Mechanisms of Activation of ASMase
One mechanism for activation of ASMase involves its phosphorylation by PKCS at Ser508 which mediates UV light-induced ASMase activation and cell death in MCF-7 breast cancer cells.52 Phosphorylation of ASMase seems to be correlated with its translocation to the plasma membrane (see below) in irradiated cells.
Evidence for a Coordinated Regulation of Multiple Pathways for Ceramide Generation
A handful of studies suggest that the activity of the two SMases and the de novo pathway might be regulated in a coordinated fashion by the same agonist, resulting in the transient generation of distinct "waves" of ceramide increases that might serve to diversify the biological effects of ceramide. These studies also emphasize the significance of specific ceramide species in defined stages of cellular stress response.
For example, a transient increase of ceramide is observed within minutes after exposure to ionizing irradiation which is a consequence of DNA damage-independent acid SMase173 or neutral SMase activation.47 Several hours after irradiation, a second wave of ceramide accumulation is observed depending on the DNA damage-dependent activation of ceramide synthase. It seems that the late ceramide accumulation is also dependent on the first one and is rate limiting for the apoptotic process induced by irradiation.173
Kroesen et al174 on the other hand, showed that cross linking of the B-cell receptor generates C16-ceramide upstream of the mitochondria in a caspase independent manner and that inhibition of C16 ceramide generation rescues from cell death.56 C24 ceramide however was generated downstream of mitochondrial dysfunction in a caspase dependent manner. All of the increases are seemingly due to activation of de novo synthesis and apparently different members of the ceramide synthase family might be differentially regulated.
A classical example of coordinated regulation of neutral and acidic SMase is the early work of Kronke and colleagues.175 In these studies, stimulation with TNFa lead to activation of both enzymes through apparently separate mechanisms since different domains of the cytosolic tail of the TNFa receptor were responsible. Furthermore, while the activation of ASMase was linked to NFkB activation, NSMase seemed to regulate the activation of proline-directed serine/ threonine protein kinase(s).
Mechanisms of Ceramide Effects on Cellular Functions
Ceramide-Interacting Molecules
PKCt, (reviewed in ref. 114)
PKC atypical PKC isoform, was identified as a molecule that ceramide directly binds and activate. Notably, ceramide-induced activation of PKCt, is linked to inhibition of Akt-1. Akt-1 is a key regulatory molecule for various metabolic cellular functions, cell proliferation and cell death, which has been known for a long time to be inhibited by ceramide. Ceramide-activated PKCt, seems to phosphorylate Ser34 of the Akt-1 pleckstrin homology domain, thus preventing the interaction of Akt-1 with PIP3 and respectively with the plasma membrane.176-178 In addition to stimulating the kinase activity of PKC^, ceramide binding seems to affect the ability of PKCC to interact with other proteins. In vascular smooth muscle, ceramide stabilizes the interaction of Akt-1 and PKCt, within caveolin-enriched lipid microdomains to inactivate Akt and specifically reduces the association ofPKCt, with 14-3-3, a scaffold protein localized to less structured regions within membranes.179 In differentiating embryonic stem cells, ceramide binding to PKCt, similarly leads to activation of its kinase activity but also increases PKCt, binding to its inhibitor protein, prostate apoptosis response-4 (PAR-4), thus compromising the antiapoptotic activity of PKCC and inducing apoptosis.180
PP2A (reviewed in ref. 181)
Long chain D-erythro-C18-ceramide has been shown to activate PP2A (more specifically the AB’C trimer), PP2AC and PP1-yC and -aC in vitro suggesting a direct and stereospecific effect of ceramide on the phosphatase activity. This ability of ceramide to activate PP2A was later found to mediate the ceramide effects on various substrates relevant to the induction of apoptosis, growth arrest or inflammation, including c-Jun, Bcl-2, Akt/PKB, Rb, PKCa, ERK1/2, IRAK-1, SR proteins and many others.
Cathepsin D
Ceramide specifically binds and activates the endosomal acidic aspartate protease cathepsin D. Direct interaction of ceramide with cathepsin D results in autocatalytic proteolysis of the 52 kDa procathepsin D to the enzymatically active 48/32 kDa isoforms that can subsequently cleave and activate the apoptotic regulator Bid.182 Studies in ASMase deficient cells strongly suggest that ASMase activity is responsible for the generation of ceramide that can activate the protease.183 This is in contrast to the ability of ceramide to activate PKCt, and PP2A, which seemingly requires activation of the de novo pathway or NSMase.
Indirect Targets of Ceramide
Modulators of Apoptosis (reviewed in ref. 184)
The members of the Bcl-2 family of proteins are essential modulators of apoptotic cell death following genotoxic and nongenotoxic stress. Strong evidence links ceramide to the regulation of two members of that family, the antiapoptotic Bcl-2 and the pro-apoptotic Bax. The mechanisms involved are far from understood and apparently quite complex. TNFa- and etoposide-induced activation of NSMase leads to apoptosis via a pathway where ceramide is upstream of the antiapoptotic member ofthe family, Bcl-2 and lead to its inhibition (5).185 These effects seem to involve PP2A. Bcl-2, whose phosphorylation at Ser70 is required for its anti-apoptotic function, becomes dephosphorylated in response to ceramide in a PP2A-dependent manner and consequently is degraded in the protea-somes.186 In turn, gemcitabine-induced ceramide generation is found to enhance the expression of proapoptotic Bcl-x.187 Ceramide accumulation is also linked to the activation of the execution caspases, but it is downstream of the initiator caspases.188,189
C16-ceramide, which is generated by ASMase in response to irradiation is shown to induce a conformation change of the pro-apoptotic member Bax leading to its activation and cytochrome C release.190 It was suggested that only the production of ceramide in the mitochondria can induce the oligomerization ofBax that drives cell death.191 As Bcl-2, Bax is also regulated by ceramide via PP2A, since its dephosphorylation is associated with conformational change and release of cytochrome C from the mitochondria. Ceramide generation by ASMase has been suggested to mediate cell death by caspase- dependent and independent mechanisms depending on the death stimulus.192
Regulators of Cell Cycle
Dephosphorylation of the Retinoblastoma protein, activation of the cyclin dependent kinase inhibitor p21 and inhibition of the cyclin dependent kinase 2193,194 are essential steps in the pathway leading to cell cycle arrest in response to ceramide accumulation. Ceramide induced-dephosphory-lation of Retinoblastoma protein is mediated through PP2A, for which Retinoblastoma protein is a direct substrate. The hypophosphorylated Retinoblastoma protein binds and sequesters E2F, an essential factor for progression through the cell cycle. The senescent-associated growth may be attributed to a defect in the phospholipase D/protein kinase C (PLD/PKC) pathway and ceramide can inhibit both PLD and PKC.195,196
Regulators of Inflammation
Studies by Hannun and Brenner were the first to show that TNFa activates the stress-activated protein kinases JNKs, resulting in the stimulation of AP-1-transcription factor and induces the translocation of NFkB to the nucleus, resulting in the stimulation of NFKB-dependent gene transcription, through ceramide.197 Ceramide inducedJNK activation has also been reported in response to FAS activation,198 irradiation199 and many cytokines. The activation ofJNK by ceramide involves Rac-1200 in the case of radiation-induced apoptosis, PKCt, in response to IL-1P201 and TAK-1 in response to TNF-a, IL-1 P, or anti-Fas antibody.202 In hepatocytes, nSMase2-generated ceramide has been shown to modulate the effects of IL-1 P on JNK phosphorylation and activity in PP2A and IRAK-1-depndent manner.
Ceramide-mediated JNK activation inhibits differentiation of skeletal muscle progenitor cells to myoblasts203 and induces the expression ofvarious acute phase proteins like Insulin-like Growth Factor Binding Protein-1 in liver.204 JNK activation mediates apoptosis in neurons in response to ceramide and Amyloid P, the protein involved in the ethiology of Alzheimer’s disease. In that system, an inhibition of NSMase was found to attenuate Amyloid P-inducedJNK phosphorylation and AP-1 DNA binding activity suggesting the involvement of the plasma membrane-associated SMase activity.205
Ceramide Effects on Membrane Organization
Some of the biological responses to ceramide generation are probably due to the effects ceramide has on the structure of membrane rafts and caveolae, on the membrane curvature and the membrane permeability to aqueous solutes. The two hydroxyl groups, the amid group protons and the trans-double bond in ceramide molecule, together with the lack of large hydrophilic head group determine the ability of ceramide to affect the properties and organization of biological membrane. The long-chain base and long saturated N-acyl chains accompanied by a very small hydrophilic head promote the partitioning of ceramide into ordered membrane domains. Together with SM, which has a strong affinity for interacting with membrane cholesterol, ceramide participate in the formation of the caveolae, the signaling platforms on the cell surface. While SM is by far much more abundant than ceramide in the lipid rafts, the generation of ceramide within rafts dramatically alters the biophysical properties of these membrane domains. Ceramide molecules have the tendency to spontaneously self associate to small ceramide-enriched membrane microdomains.206 Gulbins and colleagues had proposed that these ceramide microdomains spontaneously fuse to form large ceramide-enriched macrodomains or platforms. The formation of such platforms is proposed to underline the process of agonist induced receptor clustering and consequently, the reorganization of intracellular signaling molecules to transmit a signal into the cell. Among the receptors affected by ceramide-mediated aggregation/clustering are CD95, CD28, TNFa, CD40, FcgRII.207 Intriguingly, the acidic form of SMase seems to be involved in the formation of these signaling platforms, since it has been shown to translocate to the plasma membrane under certain conditions. In further support to that extend, overexpression of the SM synthase, SMS-1, which enriches plasma membrane with SM also enhances the translocation of Fas into lipid rafts leading to subsequent Fas clustering, DISC formation, activation of caspases and apoptosis, suggesting that SM levels in the lipid rafts might also be a critical factor possible by acting as a source for ceramide.208
Another intriguing property of ceramide is its ability to spontaneously form channels into planer bilayers, liposomes and biological membranes indicating that ceramide might regulate membrane leakiness. Experiments with unilamellar vesicles show that ceramide forms large pores that can allow the efflux of proteins as large as 60 kDa. Studies by Colombini and colleagues had shown that at physiologically relevant levels, ceramides form stable channels in mitochondrial outer membranes capable of passing the largest proteins known to exit mitochondria during apoptosis including cytochrome C.209 Ongoing efforts on characterizing the ceramide metabolism in this organelle will shed more light into the physiological function of ceramide in the mitochondria.
Finally, studies in cells isolated from ASMase-deficient mice or Niemann-Pick patients had shown defects in the phagolysosomal fusion and rab-4-mediated endocytosis210 indicating that ASMase regulates select vesicular fusion processes by modifying the steric conformation ofcellular membranes.211 These conclusions are supported by series of studies on the infectivity of Listeria monocytogenes and Mycobacterium avium discussed earlier.
Conclusion
It was realized long ago that sphingolipid structure is highly diverse and encodes distinct chemical information that can be conveyed during cell signaling.212 Research spanning through the last two decades established experimentally the role ofthis novel family of bioactive lipids in signaling. Ceramide emerged as the key metabolite in several sphingolipid-based signaling networks and was recognized to mediate conserved pathways of cellular stress response. With the genetic cloning and characterization of the majority of ceramide metabolizing enzymes, it also became clear that ceramide metabolism is finely tuned leading to spatial, temporal and species-specific accumulation during stress. A strict substrate specificity and distinct cellular localization of the enzymes that catalyze ceramide synthesis and degradation has brought diversity in the signaling molecules and cellular responses regulated by ceramide. The distinct ability of ceramide to alter the biophysical properties of cellular membrane adds additional layer of complexity in our understanding of the roles of ceramide in cellular stress response.