1. Introduction
In line with the rapid development of miniaturized and chip-integrated approaches to analytical and bioanalytical systems, the proteomics work scheme has also been the focus of dimensional reduction. The driving arguments for miniaturization are seen in the possibilities of improved sample throughput, reduced sample volume requirements, lower reagent costs, and improved limits of detection (Reyes et al., 2002; Auroux et al., 2002). In recent years, the analytical endpoint in proteomics has to a great extent become dominated by mass spectrometry and database matching. In this time, laser desorption ionization (MALDI) and electrospray ionization (ESI) have become the two major techniques to ionize the target species prior to time-of-flight separation based on mass and charge (James, 2000). The aspects on miniaturization in this field have, to a great extent, taken the course of optimizing the sample handling and processing steps prior to the MS readout (Marko-Varga et al., 2004).
2. Miniaturization in MALDI-TOF MS
An inherent bottleneck in MALDI-TOF MS has been the transfer of the proteomic sample from, for example, a microtiter plate format to the mass spectrometry target plate. The MS instrumentation was, and still most are, not equipped with an MS target plate loader that can handle the dimensions of a 96-well format. As the industry sample-handling robotics follows the dimensions of the 96-well standard format, a sample transfer and format change from the well plate to the MALDI-target is a request. Recent developments have demonstrated that miniaturized protocols to deposit proteomic samples may provide improved analytical performance in the proteomic work scheme such as increased sample throughput (Ekstrom et al., 2000; Little et al., 1997), continuous or semicontinuous MALDI-readout from micro liquid chromatography separations (Preisler et al., 1998; Preisler et al., 2002; Miliotis et al., 2000; Wall et al., 2002).
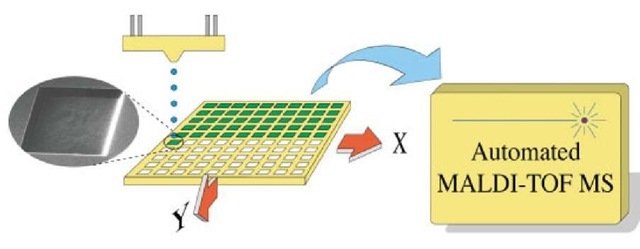
A general strategy employed in the protocols for sample handling on the MALDI target has followed the findings reporting that reduced sample spot size provides increased sensitivity in the MS readout (Ekstrom et al., 2000; Gobom et al., 1999; Onnerfjord etal., 1998). This has also been realized in commercial systems such as the MicroMass, MassPrep™ MALDI-Target, and the so-called anchor point MALDI targets by Bruker Daltonics GmbH, Germany (Schuerenberg et al., 2000). Work on further miniaturization and controlling the confinement of the sample to a predefined spot was demonstrated by Ekstrom et al. (2001a) using in-house developed piezoelectric dispensing for controlled deposition of the sample and also silicon microfabricated MALDI target plates to confine the deposited fluid by means of surface tension forces in the so-called on-spot enrichment. Later, this was also demonstrated on disposable polymer nanovial target plates (Marko-Varga et al., 2001; Ekstrom et al., 2001b). Figure 1 shows the principal setup for piezoelectric sample deposition and confined sample deposition in nanovial target plates. More recently, a stand-alone system for automatic sample transfer from chip-integrated solid-phase extraction microarrays has been realized (Wallman etal., 2003). The sample is transferred from the solid-phase microextraction chip to the microdispenser solely by means of capillary forces (i.e., no external pumping is needed to drive the sample flow through the microchips for subsequent piezoelectric deposition onto a MALDI target plate). The chip-based sample handling protocol provides both sample enrichment and cleanup performed in two steps; (1) microchip solid-phase enrichment and (2) on-spot enrichment, that is, a dual-enrichment mode is obtained that offers a sensitivity increase, superseding conventional MALDI sample preparation protocols (Wallman et al., 2004).
Figure 1 Principal setup for nanovial sample deposition prior to MALDI-TOF MS readout
3. Miniaturization in ESI-MS
Interfacing chip-integrated protocols to electrospray ionization mass spectrometry (ESI-MS) has been a long-sought goal and several promising approaches have emerged lately. A key issue in this development has been the microfabrication of an electrospray tip that provides both the necessary microfluidic and electrostatic features. The early chip/ESI work demonstrated electrospray directly from the end piece of a microchip with an embedded microchannel (Xue et al., 1997; Ramsey and Ramsey, 1997; Kameoka etal., 2001). As the flat front surface of the chip side does not provide an optimal zone for building up a reproducible Taylor cone to generate a stable electrospray, much effort has been put into the development of a microtechnology fabrication process for nanoelectrospray tips. The strategy has therefore been to limit the spreading of the effluent at the ESI-point by extending the microchannel beyond the bulk material of the chip. The insertion of a conventional capillary or regular ESI-tip at the chip termini is a common approach (Figeys and Aebersold, 1998; Bings et al., 1999), though not very amenable to low-cost mass fabrication.
With the advent of deep reactive ion etching (Clerc et al., 1998), silicon micro-fabrication could offer engineering tools for out-of-plane high-aspect ratio and high-fidelity microstructures, which basically corresponds to the geometries needed for making a good ESI-tip. Advion BioSciences Ltd., Ithaca, NY, USA, currently offers chip-based ESI-tips fabricated as fine cylindrical pipes in an array format using silicon micromachining (Van Pelt et al., 2003a,b). This design has proven to be reproducible and sufficiently robust for automated ESI applications. A similar design was later proposed by the group of Stemme, presenting a slight modification of these tips with a reduced front surface area and thereby claiming improved ESI conditions (Griss et al., 2002). Silicon microfabricated devices do not offer single-use components owing to the production costs associated with clean room manufacturing and, consequently, much interest has lately been put into the development of disposable polymer ESI-tips. This development also follows the trends of biochip developments where low-cost polymer chips have gained much attention recently (Ng et al., 2002). In line with this, Craighead et al., proposed a polymer-based chip-integrated ESI interface based on a triangular polymer lip extending from the microfluidic chip, guiding the sample from the exit of the chip to the point of Taylor cone formation (Kameoka et al., 2002). This approach seems like a very reasonable and low-cost solution to the problem of controlling the fluid in the high-voltage field at the chip exit of ESI. An alternative ESI interface, equally interesting from a scientific as well as commercial point of view was demonstrated by Killeen et al., Agilent Technologies Inc., Paolo Alto Ca, USA, with a complete system fabricated in laminated polyimide sheets that holds both a micro LC column and upstream interface to conventional rotary valves for sample injection and eluent control (Killeen et al., 2003). The chip outlet is defined as a fine laser-cut tip at the end of the separation channel, providing a robust and simple ESI interface. It should be noted that this design offers both on- and off-chip interfacing, which commonly is not the case in regard to lab-on-a-chip systems. This means that conventional high-fidelity analytical techniques can be linked upstream to the chip-integrated sample processing.
4. Conclusions
Ongoing research within miniaturization strategies of mass spectrometry sample handling and processing indicates that we currently are in a transition stage where commercial initiatives at an increasing pace now are entering the arena. The field of lab-on-a-chip, micro total analysis systems, BioMEMS, and nanobiotechnology have now reached a level of maturity where the major interdisciplinary bridging of knowledge is at hand. Also, fundamental material science developments have advanced sufficiently to provide the fruitful environment needed to be able to successfully address the questions raised in a bioanalytical situation utilizing MEMS and nanotechnology principles and methodologies. It can be anticipated that we are facing a prosperous future with rapid and dramatic improvements in sample preparation, and handling for mass spectrometry on the basis of novel miniaturized concepts, offering both improved sample throughput, sensitivity, and reduced sample volume requirements.