Afferent Connections of the Cerebral Cortex
The Thalamus
Defining Characteristics of Thalamic Nuclei
In brief, the major thalamic nuclei can be divided into the following groups: anterior, medial, lateral, midline, and intralaminar nuclei. In addition, the terms specific, association, and nonspecific nuclei have also been used to functionally define and characterize the thalamic nuclei. These terms are defined in the following sections. Specific Thalamic Nuclei. The characteristics of specific thalamic nuclei include the following: (1) they receive specific signals from sensory pathways such as the inferior colliculus (auditory), retina (visual), medial lemniscus, and spinothalamic and trigeminothalamic tracts (somatosensory); and (2) they project topographically to specific regions of the cerebral cortex (e.g., temporal neocortex, visual cortex, and primary somatosensory cortex). Thus, the function of these nuclei is to relay specific sensory information originating in the periphery to a specific region of the neocortex.
Association Nuclei. Association nuclei receive few, if any, afferents from ascending sensory pathways. They do, however, receive inputs from regions such as the cerebral cortex, limbic nuclei, basal ganglia, and nonspecific thalamic nuclei. Association nuclei also project to specific regions of the cerebral cortex. Their functions appear to be complex and integrative in nature and, in many cases, are not entirely understood. Figure 26-5 depicts the anatomical arrangement of the thalamic nuclei and their output relationships with the cerebral cortex. Nonspecific Thalamic Nuclei. The unique feature of nonspecific thalamic nuclei is that they appear to project to wide regions of cerebral cortex rather than to localized areas like the specific and association nuclei. Specific and association nuclei generally project topographically to specific regions of the cerebral cortex. The projections from nonspecific nuclei to the cortex are not topographically organized. Nonspecific thalamic nuclei also possess another feature: They project to specific thalamic nuclei. In this manner, they are capable of modulating the activity of wide numbers of cortical neurons either from direct projections to various regions of cortex or, indirectly, through their actions on different specific and association nuclei of the thalamus, which then project to their respective target regions of cortex. Inasmuch as monoaminergic (and other) axons of the reticular formation project to nonspecific thalamic nuclei, these direct and indirect connections between nonspecific thalamic nuclei and cortex provide important substrates by which the reticular formation can significantly alter cortical excitability levels.
Two other general features concerning thalamocortical relationships are to be noted. The first is that, because the nonspecific thalamus projects to wide areas of cortex, most regions of cortex thus receive converging inputs from both specific (or association) nuclei and nonspecific thalamic nuclei. Specific and association thalamic nuclei project most commonly to layers III and IV of the neocortex, whereas the projections of nonspecific thalamic nuclei may reach more superficial layers, including layer I (Fig. 26-4). Because such an arrangement makes it possible for regions such as the reticular formation to alter cortical excitability levels, they further serve to increase (or decrease) the likelihood that cortical neurons will respond more effectively to other inputs from specific or association thalamic nuclei.
The second feature is that specific, association, and nonspecific thalamic nuclei share reciprocal connections with the cerebral cortex. The apparent significance of such reciprocal connections is that they provide the basis for a feedback mechanism from the cortex to the thalamus, which ultimately serves to control the amount of information that reaches a specific region of cortex at any given moment.
FIGURE 26-5 Thalamocortical relationships. (A) The relative positions of thalamic nuclei. (B) Lateral (left) and medial (right) views of the cerebral cortex that demonstrate the projection targets of thalamic nuclei. Color-coding is to facilitate visualization of the projection targets of thalamic nuclei on the cerebral cortex. VPM = ventral posteromedial nucleus; VPL = ventral posterolateral nucleus; VA = ventral anterior nucleus; VL = ventrolateral nucleus.
Functional Organization of the Thalamus Specific Thalamic Nuclei
Somatosensory Relays. The primary receiving areas for inputs from the body associated with touch, pressure, and movement at the joints (i.e., conscious proprioception) is the ventral posterolateral nucleus (VPL). The VPL receives afferents fibers from the medial lemniscus and spinotha-lamic tracts.Fibers arising from the medial aspect of the VPL project to the arm region (lateral aspect) of the postcentral gyrus, while the lateral aspect of the VPL projects to the leg region of the postcentral gyrus (midline region [Figs. 9-7 and 26-6]).
The ventral posteromedial nucleus (VPM) receives similar somesthetic kinds of inputs from the trigeminotha-lamic tract. The VPM, in turn, projects its axons to the head region (far lateral aspect) of the postcentral gyrus. Collectively, the VPL and VPM are referred to as the ventral basal complex and contain all the ascending somatosensory inputs from the whole body (including the head region).
Pain Relays. The spinothalamic and ventral trigeminoth-alamic tracts mediate ascending pain signals to the thala-mus from the body (cell bodies for tract fibers lie in the contralateral proper sensory nucleus [lamina IV] of the spinal cord) and head (contralateral spinal trigeminal nucleus of the lower brainstem), respectively. The primary targets of these signals are the posterior thalamic complex (which is composed of several different nuclear groups) and the intralaminar nuclei (such as the centromedian and parafascicular nuclei), although some fibers also terminate in the VPL and VPM of the thalamus. It could be that the more affective components of pain are directed to the posterior thalamic complex and intralaminar nuclei.
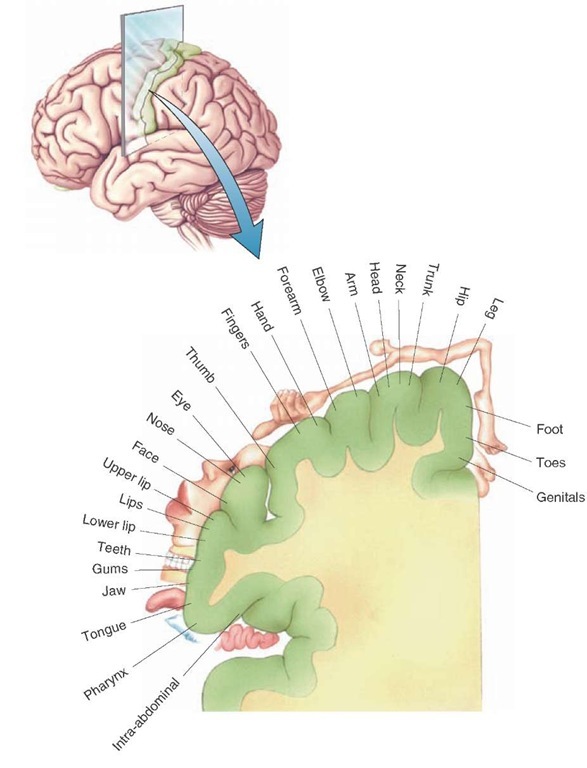
FIGURE 26-6 Cortical homunculus: somatotopic organization of the body surface as represented on the postcentral gyrus of the cerebral cortex. Neurons situated along the medial-to-lateral surface of the postcentral gyrus are responsive to stimulation of a specific aspect of the body region as indicated in this illustration.
Our understanding of the functional organization of the posterior thalamic complex is made more complex by the fact that this nucleus also integrates other somatosensory and auditory signals. The precise locations of pain inputs within the cerebral cortex have not been clearly localized. However, studies conducted in cats and monkeys suggest that cortical neurons that respond most clearly to nocicep-tive stimuli lie in a region called the "second somatic area" and the area immediately caudal to it. In humans, these regions would approximately correspond to the supramar-ginal gyrus and insular region.
Thermal Relays. The spinothalamic tract mediates thermal signals from the spinal cord to VPL. Thermal signals are then transmitted to the postcentral gyrus, but the sites where these fibers are distributed do not appear to be localized to any one area of the postcentral gyrus.
Auditory Relays. The medial geniculate nucleus is the tha-lamic relay nucleus that receives inputs from the inferior colliculus via the brachium of the inferior colliculus. The pathway is tonotopically organized (i.e., different groups of neurons respond to different sound frequencies) at both brainstem levels as well as in the medial geniculate nucleus. The medial geniculate nucleus projects mainly to the transverse temporal gyri of Heschl (Brodmann’s area 41), which is the primary auditory receiving area. Additional auditory fibers project to the surrounding auditory association area (Brodmann’s area 42 [Fig 26-3]).
Visual Relays. The primary visual relay nucleus in the tha-lamus is the lateral geniculate nucleus.The retinal inputs to the lateral geniculate nucleus on the left side of the brain represent the right hemifield, and the left hemi-field is represented on the right lateral geniculate nucleus. Neurons located in both magnocellular (l ayers 1 and 2) and parvocellular (layers 3-6) regions of the lateral geniculate nucleus project to the primary visual cortex (area 17).
Taste Relays. As indicated earlier, parts of the VPM receive somatosensory inputs from the trigeminal system. Other groups of neurons within this nucleus, however,receive inputs from the solitary nucleus (which includes a relay in the pontine taste [parabrachial] nucleus). These inputs mediate taste signals from the periphery. The region of VPM receiving taste inputs projects to the far lateral aspect of the postcentral gyrus bordering on the Sylvian fissure.
Vestibular Relays. The vestibular nuclei project their axons either to the cerebellum or into the medial longitudinal fasciculus. Whereas most fibers contained within the medial longitudinal fasciculus terminate on the motor nuclei of cranial nerves (CN) III, IV, and VI, some axons are believed to bypass these nuclei and reach parts of the VPL nucleus. In turn, these vestibular inputs are then relayed to the postcentral gyrus. Although the validity of these connections has been suggested, it is questionable whether the conscious awareness of vestibular sensations is specifically associated with this pathway. Instead, it might be that such inputs relate more closely to the regulation of motor functions and that the conscious awareness of ves-tibular signals may reside elsewhere in the cortex.
Olfactory Relays. Of all the sensory relay systems, the olfactory pathway is the only one that can reach the cerebral cortex without having to synapse in the thalamus.However, a parallel pathway by which olfactory signals can reach the cerebral cortex via the thalamus also exists. This pathway involves amygdaloid and prepyriform cortex projections to the mediodorsal thalamic nucleus, which, in turn, project to wide areas of the anterior aspect of the frontal lobe, including the prefrontal cortex.
Association Nuclei
Nuclei Mediating Motor Functions. Different portions of the ventral anterior (VA) and ventrolateral nucleus (VL) receive inputs from structures associated with motor functions. These projections arise from the cerebellum and basal ganglia. Specifically, fibers arising from the dentate nucleus of the contralateral cerebellum project to the posterior part of the VL. The anterior part of the VL receives inputs from the medial pallidal segment and substantia nigra. These two components of the basal ganglia also project to the VA. The projections to motor regions of the cortex from these regions are as follows: The VL projects to the primary motor cortex (area 4) and to the premotor cortex (parts of area 6), and the VA projects more diffusely to the supplementary and premotor areas (area 6). Thus, the VA and VL provide the substrate by which different components of the motor systems can provide feedback signals back to the motor regions of the cerebral cortex that are essential for normal motor functions to take place. When such feedback is lost, significant motor dysfunctions ensue.Also note that parts of the VA also function as a nonspecific nucleus and produce widespread excitability changes of cortical neurons.
Nuclei Mediating Functions Associated With the Limbic System. The connections of the anterior nucleus of the thalamus contribute to part of the Papez circuit (see Fig. 25-4). The anterior nucleus receives inputs from the subicular cortex of the hippocampal formation and from the mammillary bodies via the mammillothalamic tract. The anterior nucleus, in turn, projects its axons to the cingulate cortex. The cingulate cortex projects to the hippocampal formation, thus completing the circuit referred to as the Papez circuit.However, more recent studies have suggested that the Papez circuit may also be associated with memory functions.
Aside from inputs from the posterior parietal cortex, known inputs to the lateral dorsal nucleus have not been clearly defined but are thought to arise from several limbic nuclei (i.e., septal area and hippocampal formation). The outputs of the lateral dorsal nucleus have been shown to project to several regions of the limbic system, including the cingulate cortex, and to the medial wall of the posterior parietal cortex. Its functions remain obscure.
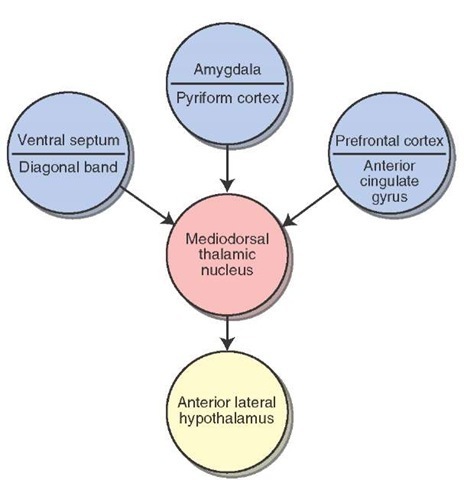
The mediodorsal thalamic nucleus receives converging inputs from a number of limbic structures (Fig. 26-7). These include the amygdala, prepyriform area, septal area, anterior cingulate gyrus, and prefrontal cortex. The medi-odorsal thalamic nucleus has two important projections. The first is a massive distribution of fibers to the prefrontal cortex and to adjoining regions of the frontal lobe. The second is a projection to the midline thalamus that forms the first limb of a polysynap-tic pathway to the anterior lateral hypothalamus. The likely function of these converging inputs to the medio-dorsal nucleus is that it provides an additional substrate by which limbic structures can modulate hypothalamic processes.
FIGURE 26-7 The mediodorsal thalamic nucleus. Schematic diagram illustrating the converging limbic inputs on the mediodorsal thalamic nucleus and its output onto the hypothalamus.
Evidence that this nucleus also mediates affective components of pain comes from clinical reports that revealed that surgical lesions of the mediodorsal nucleus have sometimes been carried out to alleviate the severity of certain forms of pain. Patients subjected to these surgical procedures report that they still physically experience pain but show little of the distress that is normally associated with severe pain.
Nuclei Associated With Complex Integrative Functions. The lateral posterior thalamic nucleus receives inputs mainly from the parietal lobe. Subcortical projections to this nucleus have not been clearly identified. The major cortical projection target of the lateral posterior nucleus is the superior parietal lobule (areas 5 and 7). Recall that these regions are associated with complex sensorimotor integration. They receive polysensory inputs from other sensory regions of cortex, and their fibers project to the premotor and supplementary motor areas (area 6). These regions are believed to help program the sequences of motor responses involved in complex motor processes. Accordingly, by providing significant inputs to the posterior parietal cortex, it is likely that the lateral posterior nucleus also contributes to the functions of areas 5 and 7.
The pulvinar primarily receives inputs from visual (from the superior colliculus and visual cortex) and auditory (from the temporal neocortex) regions of the brain. Its projections are directed to a wide expanse of the inferior parietal lobule and to adjoining regions of the temporal lobe. It appears that the pulvinar integrates auditory and visual inputs and, perhaps, some somesthetic impulses. It is thought to mediate complex sensory discrimination learning and cognitive functions.
Intralaminar and Midline Thalamic Nuclei
Centromedian Nucleus.These nuclei comprise the nonspecific thalamus. The largest of the nonspecific intral-aminar thalamic nuclei is the centromedian nucleus. It receives inputs from the cerebral cortex and reticular formation, nociceptive stimuli from the spinal cord, and some fibers from the medial pallidal segment. The centromedian is the only thalamic nucleus that projects to the basal ganglia (i.e., the thalamostriate projection to the putamen). Moreover, the centromedian nucleus, like other nonspecific thalamic nuclei, projects to specific thalamic nuclei and can, thus, influence the transmission of sensory information to the cortex.
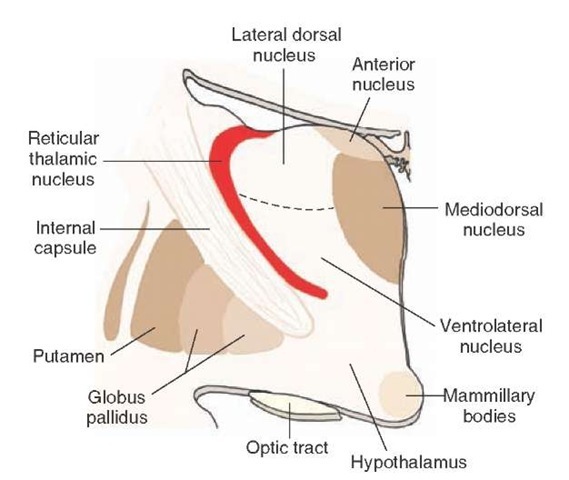
The Thalamic Reticular Nucleus. The reticular nucleus differs from other thalamic nuclei and does not readily fit the classification scheme of thalamic nuclei mainly because it does not project to the cortex. It is located on the lateral margin and dorsal aspect (within the external medullary lamina) of the thalamus (Fig. 26-8). As a result of its location, most, if not all, corticothalamic fibers pass through this nucleus and provide collateral connections with it. Thalamocortical relay neurons also provide collaterals that synapse with neurons of the reticular nucleus.
FIGURE 26-8 Reticular thalamic nucleus. The reticular nucleus of the thalamus lies just medial to the internal capsule. It differs from other thalamic nuclei in that it does not project to the cerebral cortex. Instead, it contains inhibitory neurons, which modulate thalamic neurons that project to the cortex.
Moreover, both thalamocortical and corticothalamic fibers that provide inputs to the reticular nucleus are reciprocally connected. Such an arrangement permits the reticular nucleus to evaluate the amount of information that is descending from the cortex as well as the amount that is ascending to the cortex. Adjustments to marked changes in descending or ascending inputs can be made by the reticular nucleus because its output neurons, which are Y-aminobutyric acid (GABA)-ergic, inhibit the specific thalamic nuclei with which they make synaptic contact. The interrelationships among these nuclei may be associated with states of sleep and wakefulness. Here, activation of the reticular nucleus may serve to functionally disconnect the cerebral cortex from the thalamus for periods of time when reticular neurons display bursting or tonic discharges during sleep.
Other (Nonthalamic) Regions That Project to the Cerebral Cortex
Brainstem Reticular Formation
Monoaminergic fibers arising mainly from the pons and midbrain project to wide areas of the cerebral cortex and serve as significant modulators of cortical neurons. Serotonergic fibers from the raphe neurons (mainly of the pons) project to most, if not all, regions of the cerebral cortex and reach all cell layers. Dopaminergic fibers (see Fig. 8-8) and noradrenergic fibers also project to wide areas of the cerebral cortex but in less abundant quantities than serotonin fibers (Figs. 26-9 and 26-10). The dopaminergic fibers arise from the ventral tegmental area of the midbrain. The densest quantities of fibers appear to supply motor regions and the prefrontal cortex.
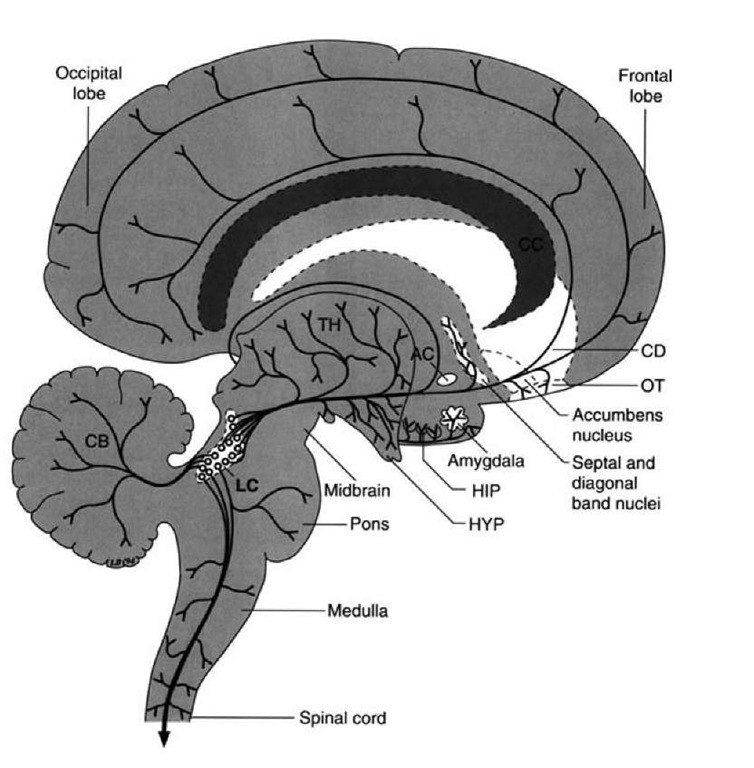
Noradrenergic fibers arising from the nucleus locus cer-uleus of the pons also project to wide areas of the cerebral cortex similar to the distributions for dopamine and serotonin.
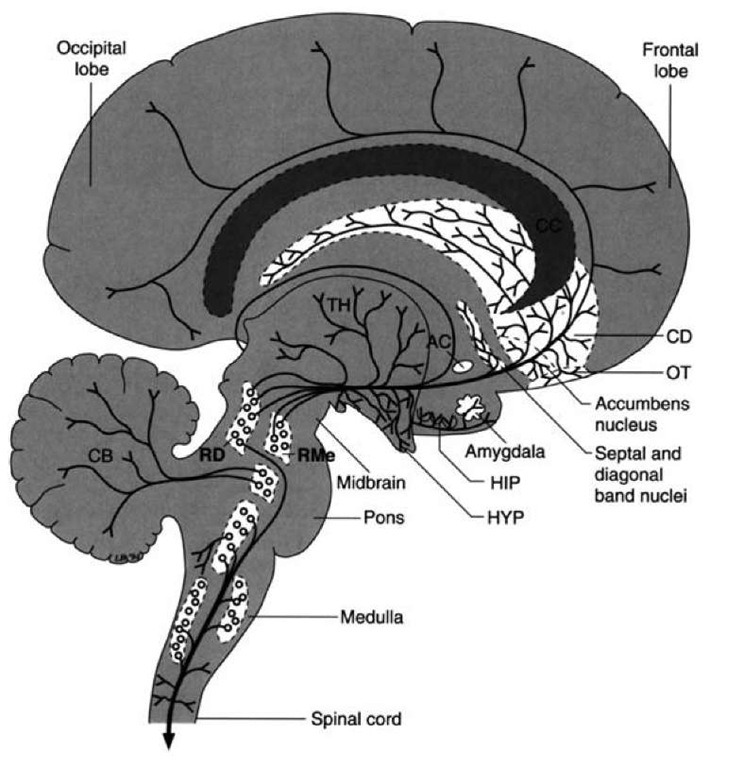
FIGURE 26-9 Serotonergic projections. Diagram illustrates the projection pathways of the serotonergic fiber systems from the raphe complex of the pons and midbrain to the cerebral cortex. Other serotonergic projections to the cerebellum, diencephalon, and spinal cord are presented for purposes of completion. AC = anterior commissure; CB = cerebellum; CC = corpus callo-sum; CD = caudate nucleus; HIP = hippocampal formation; HYP = hypothalamus; OT=olfactory tubercle; RD = dorsal raphe nucleus; RMe = median raphe nucleus.
FIGURE 26-10 Noradrenergic projections. Diagram illustrates the projection pathways from the locus ceruleus (LC) to the hypothala-mus (HYP), thalamus (TH), limbic regions including the amygdala and hippocampus (HIP), and the wide regions of the cerebral cortex. Other projections are presented for purposes of completion, including the spinal cord, medulla, and cerebellum (CB). AC = anterior commissure; CD = caudate nucleus; OT = olfactory tubercle.
Forebrain
The cerebral cortex also receives a significant contribution of cholinergic fibers from the basal nucleus of Meynert.These fibers supply cholinergic fibers to most regions of the cerebral cortex (see Fig. 8-3). Damage to these cholinergic neurons has been associated with Alzheimer’s disease.The etiology of this disease remains unknown.