Gamma Aminobutyric Acid
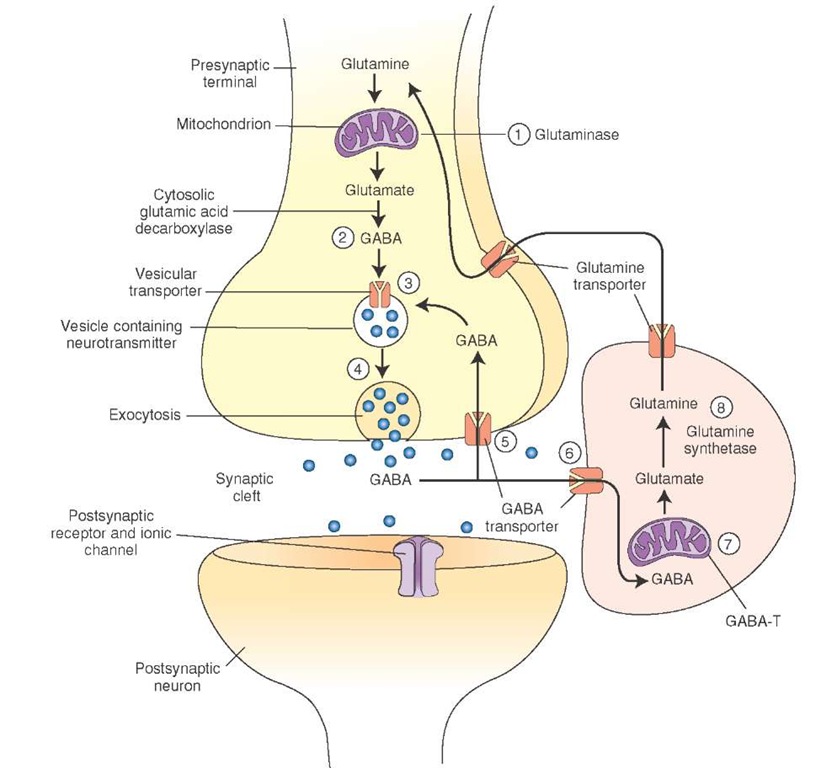
Synthesis and Removal. The following steps are involved in the synthesis of gamma aminobutyric acid (GABA) and its removal from the synaptic cleft (Fig. 8-8).
1. Glutamine is converted into glutamate by an enzyme, glutaminase.
2. GABA is formed by a-decarboxylation of glutamate. This reaction is catalyzed by a cytosolic enzyme, L-glutamic acid-1-decarboxylase (GAD), which is present almost exclusively in GABAergic neurons. GAD is not present in neurons using glutamate as transmitter or in glia. It requires pyridoxyl phosphate (a form of vitamin B6) as a coenzyme.
3. Synthesized GABA is taken up into vesicles where it is stored.
4. It is released into the synaptic cleft by exocytosis.
5. After its release, GABA is taken up into presynaptic terminal via GABA transporters and repackaged into vesicles for subsequent use.
6. GABA is also taken up into the glia via GABA transporters.
7. In glia, GABA is converted to glutamate by a mitochondrial enzyme, GABA transaminase (GABA-T).
8. Another enzyme, glutamine synthetase, converts glutamate into glutamine, which is then transported into the neighboring nerve terminals where it is processed to synthesize glutamate.
Distribution. GABA is found in high concentrations in the brain and spinal cord but is absent in peripheral nerves or peripheral tissues. Unlike glutamate, GABA is not an essential metabolite, and it is not incorporated into a protein.
Physiological and Clinical Considerations. Some of the important physiological and clinical considerations regarding this neurotransmitter are as follows.
1. GABA is an inhibitory transmitter in many brain circuits. For example, GABA is used as an inhibitory neurotransmitter by the Purkinje cells in the cerebellum. Alteration of GABAergic circuits has been implicated in neurological and psychiatric disorders like Hunting-ton’s chorea, Parkinson’s disease, senile dementia, Alzheimer’s disease, and schizophrenia.
FIGURE 8-8 Steps involved in the synthesis and release of gamma aminobutyric acid (GABA).
2. As mentioned earlier, GAD requires vitamin B6 as a coenzyme. Therefore, dietary deficiency of vitamin B6 can lead to diminished GABA synthesis. In a disastrous series of infant deaths, it was noted that vitamin B6 was omitted in an infant feeding formula. GABA content in the brain of these infants was reduced. Subsequently, there was a loss of synaptic inhibition that caused seizures and death.
3. Because epileptic seizures can be facilitated by lack of neuronal inhibition, increase in the inhibitory transmitter, GABA, is helpful in terminating them. Thus, valproic acid (dipropylacetic acid) is useful as an anti-convulsant because it inhibits GABA transaminase, which is an enzyme that metabolizes GABA, and increases GABA levels in the brain.
4. Barbiturates act as agonists or modulators on postsynap-tic GABA receptors and are used to treat epilepsy.
Glycine
Synthesis and Removal. In the nerve terminal, serine is formed from an intermediate (3-phosphoglycerate) produced by glycolysis of glucose. Glycine is formed from serine by an enzyme, serine transhydroxymethylase. This reaction is folate-dependent. After its release, glycine is taken up by neurons by an active sodium-dependent mechanism involving specific membrane transporters. Distribution. Glycine is found in all body fluids and tissue proteins in substantial amounts. It is not an essential amino acid, but it is an intermediate in the metabolism of proteins, pep-tides, and bile salts. It is also a neurotransmitter in the CNS. Physiological and Clinical Considerations. Glycine has been implicated as an inhibitory neurotransmitter in the spinal cord, lower brainstem, and retina. Glycine hyperpolarizes neurons by opening chloride channels. Mutations of genes coding for some of the membrane transporters needed for removal of glycine result in hyperglycinemia, which is a devastating neonatal disease characterized by lethargy and mental retardation.
Catecholamines
The chemical structure of catecholamines includes a catechol (a benzene ring containing two adjacent hydroxyl groups). The steps involved in the synthesis of catecholamine neurotransmitters are shown in Fig. 8-9. Individual members of this group of neurotransmitters are discussed in the following sections.
Dopamine
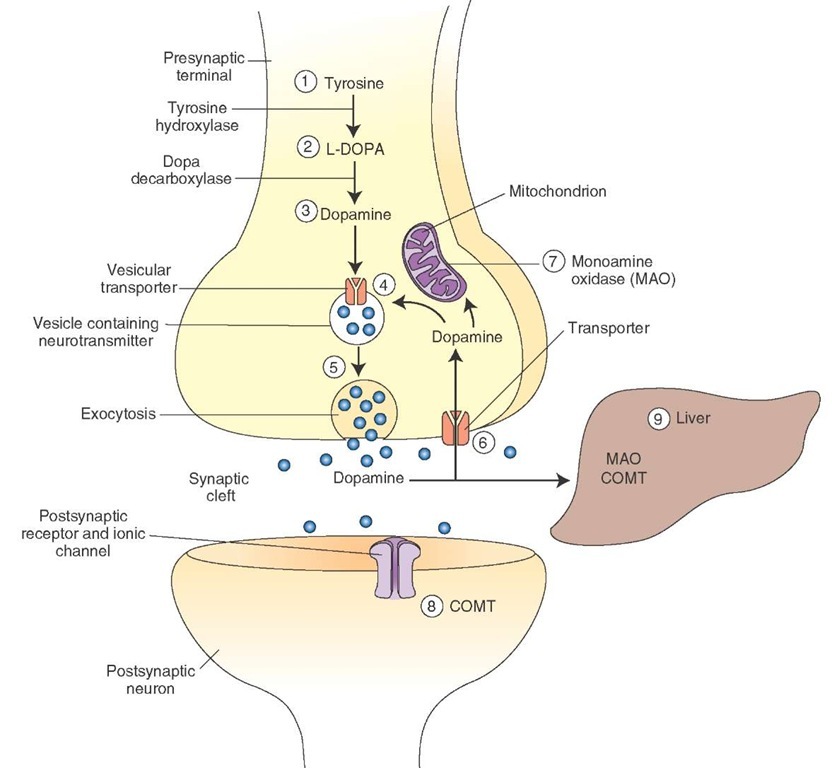
Synthesis and Removal. The steps involved in the synthesis, removal, and metabolism of dopamine are as follows (Fig. 8-10).
FIGURE 8-9 Steps involved in the synthesis of catechol-amines.
FIGURE 8-10 Steps involved in the synthesis and release of dopamine. COMT = catechol-O-methyltransferase.
1. The amino acid tyrosine (hydroxy-phenylalanine) is present in all food products and can be synthesized from phenylalanine. It enters the neuron by active transport.
2. In the cytoplasm of the dopaminergic neuron, tyrosine is converted into dihydroxyphenylalanine (DOPA) by the tyrosine hydroxylase enzyme. This enzyme is rate-limiting for the synthesis of all three catecholamines.
3. DOPA is converted to dopamine in the cytoplasm by the enzyme aromatic L-amino acid decarboxylase (DOPA-decarboxylase).
4. Dopamine is then actively transported into the storage vesicles by the vesicular transport mechanism.
5. In the dopaminergic neuron, dopamine remains unchanged in the storage vesicles and is ready for release by exocytosis.
6. Dopamine released into the synaptic cleft is actively transported back into the neuronal terminal. This process is called reuptake-1 and is the most important mechanism by which dopamine and other catecholamines are removed from the synaptic cleft. Some of the dopamine entering the neuronal terminal (about 50%) is transported into the vesicles for storage and release.
7. The remaining dopamine that enters the neuronal terminal is destroyed by a mitochondrial enzyme, monoam-ine oxidase (MAO).
8. Some of the dopamine that is released into the synaptic cleft (about 10%) is actively transported into the effector cells. This process is known as reuptake-2. Dopamine entering the effector cells is inactivated primarily by an enzyme, catechol-O-methyltransferase (COMT). Although MAO is also present in the effector cells, its role in the inactivation of dopamine is unclear.
9. The remaining dopamine in the synaptic cleft diffuses into the circulation and is destroyed in the liver by COMT and MAO. The end products of metabolism of catecholamine are organic acids and alcohols, which are excreted in urine.
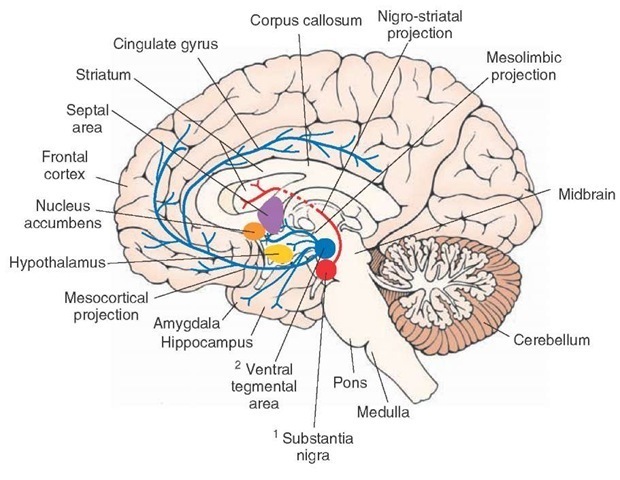
Distribution. Dopaminergic neurons are located in the following nuclei (Fig. 8-11).
1. Substantia nigra, which is located bilaterally in the midbrain. Axons of the dopaminergic neurons located in the substantia nigra (pars compacta) ascend rostrally as nigrostriatal projection, providing dopaminergic innervation to neurons located in the corpus striatum (caudate nucleus and putamen). Dopaminergic neurons in the substantia nigra are selectively degenerated in Parkinson’s disease.
2. Ventral tegmental area, which is located just medial to the substantia nigra pars compacta. Dopaminergic neurons, located in this area, project as either the mesolim-bic or the mesocortical pathway. In the mesolimbic pathway, axons of the dopaminergic neurons ascend in the brainstem and forebrain in the median forebrain bundle to supply limbic structures (amygdala, septal area, and hippocampal formation) and the nucleus accumbens (which is embedded in the ventral stria-tum). In the mesocortical pathway, axons of the dopaminergic neurons ascend in the median forebrain bundle to provide dopaminergic innervation to the frontal and cingulate cortices.
3. The hypothalamic arcuate nucleus, which is located bilaterally at the base of the third ventricle(not shown in Fig. 8-11). Dopaminergic neurons located in the arcuate nucleus project to the median eminence and release dopamine directly into the hypo-physeal portal circulation, which is then carried to the anterior lobe of the pituitary to inhibit the release of prolactin (the hormone primarily responsible for lactation).
FIGURE 8-11 Dopaminergic neurons and their projections. Axons of dopaminergic neurons located in the substantia nigra pars compacta ascend rostrally as the nigrostriatal pathway and provide dopaminergic innervation to neurons located in the caudate nucleus and putamen (striatum). Axons of another group of dopaminergic neurons that are located in the ventral tegmental area ascend in the median forebrain bundle to provide dopaminergic innervation to the frontal and cingulate cortices.
![tmp14-71_thumb[2]_thumb tmp14-71_thumb[2]_thumb](http://what-when-how.com/wp-content/uploads/2012/04/tmp1471_thumb2_thumb_thumb.jpg)