Introduction
Brain injury occurs from either a traumatic (mechanical), ischemic (decreased oxygen; accounts for 83% of stroke cases), or hemorrhagic (ruptured blood vessel; accounts for 17% of stroke cases) insult to the brain. Stroke and traumatic brain injury (TBI) are major contributors worldwide to both deaths and persistent disabilities. Stroke is the third leading cause of death (behind heart disease and cancer) in the United States, with 137,000 Americans dying from stroke each year (Heron et al., 2009). Stroke is the leading cause of serious, long-term disability in the United States. Currently, 795,000 people have a stroke each year and 15-30% of survivors have a permanent disability (Roger et al., 2011). Annually, 1.7 million people sustain a TBI in the United States, resulting in 52,000 deaths and over 124,000 permanent disabilities each year (Faul et al., 2010). Annual direct (e.g., medical) and indirect (e.g., loss of productivity) costs to the United States are $41 billion and $60 billion for stroke and TBI, respectively (Finkelstein et al., 2006; Roger et al., 2011).
Though the etiology differs between traumatic and ischemic injury, there are many similarities in their pathology (Bramlett & Dietrich, 2004; Leker & Shohami, 2002). The primary insult initiates a cascade of secondary events such as edema, excitotoxicity, and increases in free radicals, which act to spread the injury to surrounding tissue (for reviews of the pathology, see Greve & Zink, 2009 for TBI and Mitsios et al., 2006 for ischemic stroke). Note that ischemia is part of the secondary injury response for TBI (Coles, 2004; Garnett et al., 2001). The brain attempts to repair and regenerate, but depending on such factors as injury severity, age of onset, and prior injuries, these endogenous attempts are often insufficient to restore normal function. A treatment that limits the spread of secondary damage and/or promotes repair and regeneration is needed. Current clinical treatment practices for TBI primarily aim to reduce intracranial pressure in an effort to minimize brain damage caused by swelling. For ischemic stroke, the only FDA-approved treatment is breaking down blood clots with tissue plasminogen activator. However, patients must meet strict criteria for receiving this therapy, including a 4 hour time window and no evidence of the following: bleeding, a severely elevated blood pressure or blood sugar, recent surgery, low platelet count, or end-stage liver or kidney disorders. Numerous pharmacological treatments that seemed promising in animal models have failed in clinical trials (Maas et al., 2010; O’Collins et al., 2006). Patients with brain injury vary widely with respect to demographics, severity of injury, location of injury, and co-morbidity factors making clinical trials challenging. Most treatments previously tested involved pathways that are both deleterious and beneficial, making the dosage and timing critical to not interfere with normal homeostasis or reparative mechanisms in the brain. Furthermore, these treatments targeted single mechanisms, which may not be enough in light of the multi-faceted pathology. Therapies that currently seem more promising, such as progesterone administration (Wright et al., 2007) and cell transplantation, address multiple pathological events.
Mesenchymal stromal cells to treat brain injury
Mesenchymal stromal cells (MSCs)
Mesenchymal stem cells are multipotent cells that can differentiate into cells of the mesoderm germ layer. These cells can be isolated from adipose tissue, amniotic fluid, placenta and umbilical cord, though are most commonly and efficiently derived from adult bone marrow. Marrow-derived cells that adhere to tissue-culture plastic in vitro are a heterogeneous population of cells that contain mesenchymal stem cells, but the entire population is more correctly defined as mesenchymal stromal cells (Horwitz et al., 2005). As we learn more about these cell populations, the terminology evolves and the acronym MSC is used (and sometimes misused) for mesenchymal stem cell, mesenchymal stromal cell, multipotent stromal cell, and marrow stromal cell. For the purposes of this topic, we will not distinguish amongst these cell populations and use MSC as a general acronym.
Using MSCs to treat brain injury
MSCs are an attractive cell source for transplantation because they are relatively easy to obtain, expand, and manipulate in vitro. In addition, adult human MSCs do not have the tumorigenicity risks that pluripotent cells carry. Ample preclinical data demonstrate that MSC transplantation promotes functional recovery following experimental cerebral ischemic or TBI (for review, see Li & Chopp, 2009 or Parr et al., 2007). Autologous MSC therapy has already shown promise for treating clinical stroke (Battistella et al., 2011; Honmou et al., 2011; Lee et al., 2010; Suarez-Monteagudo et al., 2009) and TBI (Cox et al., 2011; Zhang et al., 2008). Collectively, these trials demonstrate that transplanting MSCs either intra-arterially, intravenously, or intracerebrally is safe and no cell-related adverse events were reported. These groups also indicate that some patients receiving MSCs had improved functional outcome; however, these hints at efficacy must be cautiously interpreted because these were primarily safety trials and were not designed to show robust efficacy.
Important considerations for using MSCs in the clinic include timing (acute versus chronic), delivery route (most commonly intravenous, intra-arterial, or intracerebral), and donor source (autologous versus allogeneic). There are advantages and disadvantages for each of these issues, which are outlined in Table 1. According to www.clinicaltrials.gov (searched in August 2011; summarized in Table 2), there are 11 ongoing clinical trials worldwide using MSCs (either primary or derivatives) to treat stroke. Of these 11 studies, 5 are using autologous MSCs and the other 6 are using allogeneic MSCs from either bone marrow, placenta (1 study) or umbilical cord (1 study). Two of the trials are injecting cells directly into the injured brain (either into the injury cavity or the peri-infarct tissue), 1 trial is injecting cells into the carotid artery, and the other 8 are injecting MSCs intravenously. With regard to timing, 2 of the trials are delivering the cells during the acute phase (within 72 hours post-stroke), 7 trials during the sub-acute phase (between 4 days and 6 weeks post-stroke), and 2 studies are delivering cells during the chronic phase (over 6 months post-stroke). As trials more definitively reveal that MSCs transplantation is both safe and effective for treating brain injury in humans, issues of delivery timing and route and donor source, as well as dosage and the use of immunosuppression will need to be more carefully compared.
|
Issue |
Options |
Advantages |
Disadvantages |
|
Timing |
Acute phase |
supports neuroprotection |
volatile environment |
|
strict timing may limit availability |
|||
|
Chronic phase |
supports regeneration |
endogenous regeneration efforts are stabilized |
|
|
easier to distinguish between effects of cell therapy and normal recovery |
|||
|
targets larger patient population |
|||
|
Delivery |
Intravenous or Intraarterial |
less invasive |
cells accumulate in the lungs and spleen |
|
cells home to site of injury |
requires high cell numbers |
||
|
possible systemic effects |
|||
|
requires blood brain barrier permeability (thus limits time window) |
|||
|
Intracerebral |
cells placed at site of injury |
more invasive |
|
|
extent and location of injury is variable |
|||
|
Donor Source |
Autologous |
immunocompatible |
patients undergo additional procedures |
|
Allogeneic |
MSCs are immunoprivileged |
may require immunosuppression |
|
|
more cost-effective |
requires storage of cell product |
||
|
better for repeat dosing |
|||
|
off-the-shelf treatment |
|||
|
cells can be manipulated ex vivo without treatment delays |
Table 1. Clinical considerations for using MSCs to treat brain injury
# Px= planned number of patients to enroll; Auto=autologous; Allo=aüogeneic; IV=intravenously; IC=intracerebral (cavity or peri-infarct tissue); IA=intra-arterial (carotid); TP=transplant
Table 2. Ongoing clinical trials for using MSCs to treat stroke
Mechanisms of action underlying beneficial effects
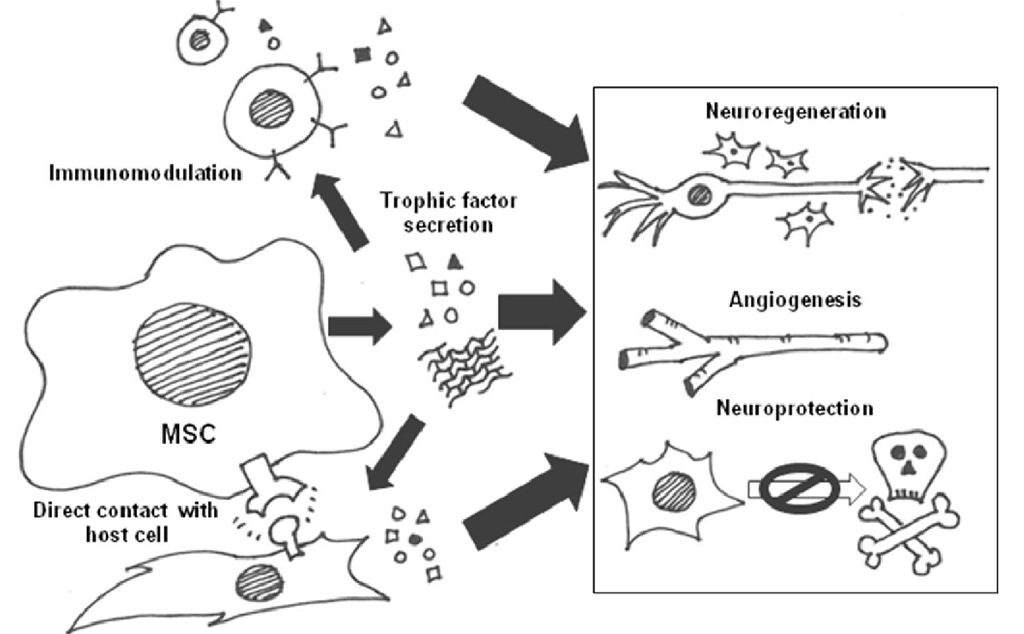
Transplanting stem cells is attractive because they can potentially differentiate into multiple cell types and replace cells lost to injury or disease. MSCs normally give rise to cells along the mesodermal lineage (including bone, cartilage, and adipose tissue); however, there are reports suggesting that they can transdifferentiate into neural cells in certain in vitro (Sanchez-Ramos et al., 2000; Woodbury et al., 2000) and in vivo (Kopen et al., 1999; Munoz-Elias et al., 2004) environments. Though some studies show a small percentage of donor MSCs express neuronal markers in the injured brain, there is little evidence that these cells functionally incorporate into the endogenous neuronal circuitry. In fact, there is a decidedly lack of evidence that neuronal replacement is the primary mechanism of action for MSC therapy; moreover, there are data demonstrating artifacts associated with MSC to neuron transdifferentiation (Barnabe et al., 2009; Lu et al., 2004; Neuhuber et al., 2004; Phinney & Prockop, 2007; Wells, 2002). There is also the possibility that MSCs replace supporting glial cells (astrocytes, oligodendrocytes, or microglia), which outnumber neurons 10:1 in the brain (reviewed in Boucherie & Hermans, 2009). However, ample evidence shows that benefits and functional recovery occur rapidly and persist long after the donor cells are gone, indicating permanent cell replacement is not required. The most likely governing mechanism is that MSCs provide trophic support to the injured brain, which augments endogenous repair and regeneration pathways. Trophic support, by definition, acts through secreted molecules called trophic factors. MSCs may act as mini-pumps delivering beneficial factors to their microenvironment. Using cells as pumps is preferred to actual engineered pumps because they can deliver a plethora of factors at the site of injury in physiologic concentrations and also respond to the needs of the injured tissue with appropriate feedback. Trophic factors can either directly or indirectly (via a mediator cell) promote neuroprotection (enhance cell survival through repair) or neuroregeneration. MSCs also secrete factors that augment angiogenesis – another important aspect of regeneration after brain injury. An additional likely mechanism of action contributing to the benefit of MSCs is immunosuppression. MSCs can affect immune cells via secreted factors, which would fall under trophic support. For the purposes of this topic, we will treat it as a separate category since targeting immune functions indirectly promotes recovery compared to acting directly on neural or vascular cells. There is a great deal of overlap between these functions and these categories are fluid. Figure 1 summarizes hypothesized mechanisms of action for MSCs in the injured brain, which are mediated by secreted factors and direct cell-cell contacts.
Terminology
Trophic support classically means to provide nutrition, but the definition has been expanded to include promoting cellular growth, survival, differentiation, or migration. Similarly, the terms "trophic factor" and "growth factor" have also become more inclusive. Neurotrophic factors are trophic factors acting specifically on neural cells, i.e., promoting the growth, survival, differentiation, or migration of primarily neurons, but also glial cells (astrocytes, oligodendrocytes, microglia and Schwann cells). The name neurotrophin is sometimes used synonymously with neurotrophic factor; however neurotrophins specify a family of four structurally-related proteins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). The term cytokines was initially used to distinguish factors that had specific immunomodulatory properties (produced by and act on immune cells), such as interleukins, lymphokines, and interferons. However, it is now known that many classic cytokines are also produced by and act on non-immune cells. Chemokines are a subclass of cytokines that promote chemotaxis (cell movement in response to a chemical concentration gradient). In general, as more functions are discovered about these proteins, definitions and classifications broaden and the terms are often used interchangeably. While trophic factors commonly refer to soluble proteins, extracellular matrix (ECM) proteins that are immobilized in the intercellular space also fall into this category since they direct cell growth, survival, differentiation, and migration.
Fig. 1. Summary of likely mechanisms of action for MSCs in the injured brain, highlighting the interconnectivity.