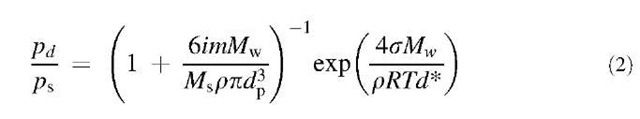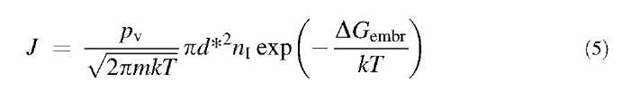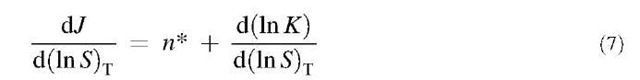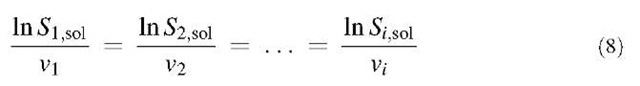In reality, there are no pure particles in the atmosphere. If the particle contains a soluble material, then during evaporation it will experience two competing effects: the Kelvin effect, which would require an increase in vapor pressure to stop evaporation, and the solute effect, in which the increase in solute concentration in the particle reduces the saturation ratio required for droplet stability. As the particle shrinks, the solute effect dominates over the Kelvin effect. The Kohler equation describes this competition:
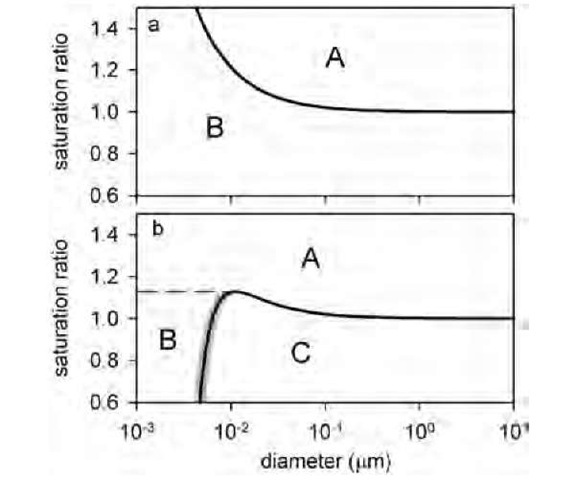
In Eq. 2, i is the solute van’t Hoff factor and m is the mass of the dissolved solute with molecular weight Ms, and p is the density of the solvent. As Fig. 7b shows, the effect of this is dramatic. A nanoparticle in a gas at a saturation ratio that places it in region A will grow indefinitely. A particle in region B will grow until it reaches the shaded part of the curve, where it equilibrates with its surroundings. A particle in region C will evaporate until it reaches the shaded part of the curve, where it again forms a stable particle. The shaded part of the curve in Fig. 7b defines a true equilibrium, unlike the remaining part of the curve and the entire Kelvin equation curve shown in Fig. 7a, both of which define points of unstable equilibrium. This equilibrium particle size will change only if the saturation ratio changes, which will move it up or down the shaded line. If a particle were to grow until it reaches the curve maximum, then it will enter region A and proceed to grow into a cloud droplet. However, if the saturation ratio were continuously lowered, the particle dries to form a solid residue. For a particle containing insoluble materials, the effect is to increase the solute effect. Effectively, the insoluble material displaces the equivalent volume of solvent. Thus for the same diameter particle, the solute concentration is larger and the solute effect is greater. It is important to note the assumptions that are used to derive Eqs. 1 and 2. These are the capillarity assumption, in which bulk thermodynamic properties (e.g., surface tension s) are assigned to particles of all sizes, and the incompressibility approximation, which states that the overall particle volume can be calculated by summing the volumes of the individual species.
Fig. 7 Saturation ratio vs. particle size for (a) pure water, using Eq. 1, and (b) water with NaCl added, assuming a dry particle diameter of 4 nm. Shaded line in (b) indicates line of stable equilibrium. Capital letters indicate regions of condensational growth and evaporation (see text).
Once a stable nanoparticle is formed, the rate at which it grows by condensation is determined by the number of random collisions of vapor molecules with the particle. The kinetic theory of gases can be applied for particle diameters smaller than the mean free path of the vapor, resulting in:
In Eq. 3, m is the mass of the vapor molecule; p is the partial pressure of the surrounding vapor; Na is Avoga-dro’s number; k is the Boltzmann constant; and pd is the partial pressure of the vapor given by the Kelvin equation (Eq. 1). Eq. 3 states that nanoparticle growth is independent of particle size—precisely the observation provided by the particle growth measurements shown in Fig. 4.
Homogeneous Nucleation
If homogeneous nucleation were to start on an individual water molecule (d*=0.4 nm), then, according to the Kelvin equation, a saturation ratio of 220 would be required. Yet experiments with pure water at 20°C have shown that the process occurs at saturation ratios greater than 3.5.[45] Clearly, homogeneous nucleation in the atmosphere does not occur by condensation of vapor on individual molecules. In reality, intermolecular forces such as van der Waals forces lead to the formation of molecular clusters. As these clusters form and fall apart, some portions of these will reach the critical cluster size, which, for pure materials, is given by the Kelvin equation (Eq. 1), and become stable nanoparticles. This is the microscopic view of homogeneous nucleation. Theoretical treatments of homogeneous nucleation fall into three categories: purely molecular theories, which apply only to simple molecules (e.g., Senger et al.[46]); phenomenolog-ical theories, which predict nucleation rates using measurable fluid properties as input; and intermediate theories, which share characteristics of each (e.g., Zeng and Oxtoby[47]). The focus of the current section will be on the phenomenological treatment classical nucleation theory (CNT) because most of our current understanding is rooted in this theory. A good reference for recent progress in models and observations of nucleation can be found in the published proceedings of a recent conference devoted to this.[48]
As in the discussion of condensation, we start with the case of homogeneous nucleation by a single species. In CNT, the energy change associated with the spontaneous formation of a critical embryo AGembr is the sum of the energy change associated with molecules leaving the vapor phase and entering the embryo and the energy associated with the curved embryo surface:
where n* is the number of molecules in the embryo, Am is the difference in chemical potential for the molecule between solution and vapor, and s is the surface tension, which is again approximated by the bulk surface tension (capillarity assumption). The nucleation rate, defined as the number of embryos nucleated per unit volume per unit time, is collision rate between molecules and embryos of the critical cluster size, multiplied by the number of critically sized embryos as given by the Boltzmann distribution. The result is:
where nI is the monomer concentration in the gas phase and m is the mass of the vapor molecule.
The chemical composition of the critical embryo can be determined by applying the nucleation theorem,[49] which relates the saturation ratio dependence on AGembr and n*:
The nucleation theorem is generally applicable to either molecular, phenomenological, or intermediate theory of nucleation. Combining Eqs. 5 and 6, one finds:
where K is the preexponential factor in Eq. 5. The d(ln K)/ d(ln S)T term can be generally neglected,[50] leaving a simple way of determining the critical cluster size n from observations of J and S.
The extension of CNT to multicomponent nucleation is straightforward[51] and involves solving the simultaneous equations:
where Si,sol is the saturation ratio of species i with respect to the liquid solution and vi is the partial molecular volume of species i. Once the mole fraction of each compound xi in the critical cluster is found from vi, the embryo diameter is calculated from:
AGembr can then be found by substituting d* into the multicomponent form of Eq. 4, in which nAm is replaced by the sum of all niAmi. This can then be used to calculate J by using a multicomponent form of Eq. 5.[52]
The principal limitation of CNT is its reliance on the capillarity assumption. For binary nucleation, it has been found that the predictions of CNT are reasonable when the nucleating species form ideal solutions;[50] however, when one of the components tends to concentrate on the surface, the theory can yield unphysical results.[53] Most intermediate nucleation theories address this issue by retaining most of CNT while introducing correction factors for the nonideal behavior of molecular clusters. Another modification to CNT is required when the nucleating species readily hydrates (i.e., binds with one or more water vapor molecules). The most important example of this in the atmosphere is sulfuric acid/water binary nucleation, which will be discussed later.
Coagulation
A brief discussion shall be devoted to coagulation, as it is one of the primary loss mechanisms for nanoparticles (Fig. 6). Coagulation is the process by which particles collide with one another and adhere to form larger particles. Nanoparticles are particularly susceptible to loss by Brownian coagulation, in which collisions are caused by relative Brownian motion. A simplified theory of coagulation[45] states that the rate of change of the number concentration, N, for particles of a given size is expressed as
K0 is the coagulation coefficient, and is a function of diffusion coefficients and diameters of each particle in the mixture. Two interesting observations emerge from coagulation theory: First, the N2 dependence in Eq. 10 means that coagulation is rapid when particle number concentrations are large. The second observation, which comes from the analysis of K0, is that coagulation rate is at its minimum when particles are all of the same size, and rises rapidly when larger particles are added to the mixture.
Observations of Atmospheric Nanoparticle Nucleation and Growth
In a recent review of more than 100 investigations of particle nucleation and growth rates, it was found that the formation rates of 3 nm diameter particles (the minimum detectable size) are in the range of 0.01 – 10 cm-3 sec-1 in the clean remote troposphere, but can be up to 100 cm-3 sec-1 in urban areas and as high as 105 cm-3 sec-1 in certain coastal areas and in industrial plumes.[15]
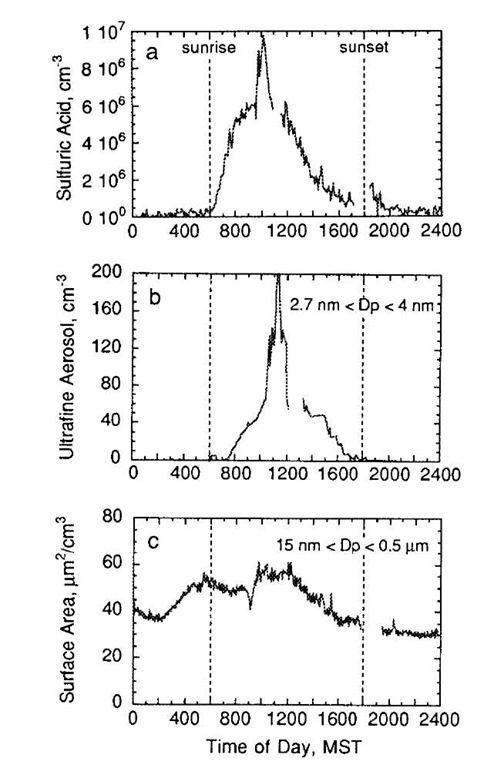
Fig. 8 Measured (a) H2SO4, (b) nanoparticle concentration, and (c) aerosol surface area concentrations at Idaho Hill, CO, a remote forest site.
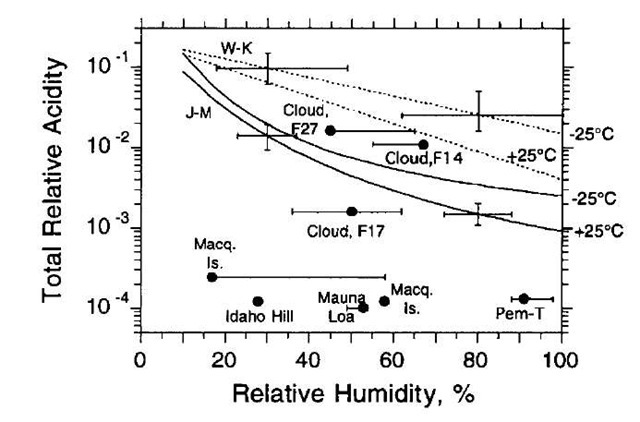
Sulfuric acid is the species most commonly associated with nucleation in the atmosphere, although there is still no direct proof of this because the chemical composition of nucleating clusters has, thus far, not been measured. Observations at a remote site in Colorado (Fig. 8), show clear correlations between increases in sulfuric acid vapor and nucleation. This frequently observed phenomenon and sulfuric acid’s extremely low volatility have made it the most likely candidate for study. Sulfuric acid forms hydrates that tend to decrease its effectiveness as a nucleating agent by a factor of 105-106, compared with the absence of hydrates.[1] Models that account for hydrate formation do so by treating hydrates as small liquid droplets.[55] When these models are applied to observations of nucleation, results such as those shown in Fig. 9 are commonly observed. In Fig. 9, conditions that initiated nucleation are plotted for a variety of sites in the troposphere. These observations are compared with two different CNTs that included corrections for hydrate formation. The plot shows basic agreement with binary nucleation theory for events observed in and around clouds, and discrepancies of two to three orders of magnitude for terrestrial locations. In these locations, not only does binary CNT inadequately predict the concentrations needed for nucleation, it also cannot model the functional dependence of nucleation rate on sulfuric acid and water concentration. Weber et al.[56] found that the formation rate is proportional to the first to second power of H2SO4 concentration, which, according to Eq. 7, indicates that the critical embryo contains only one or two sulfuric acids.
The current understanding of atmospheric nucleation, which has evolved from measurements such as those shown in Figs. 8 and 9, is that, with the exception of a small set of observations from very clean locales, binary nucleation theory does not adequately model the observed sulfuric acid concentrations that initiated nucleation. Because the observations where the discrepancy occurs were at or near ground level, this suggests that one or more of the following may be occurring: 1) nucleation is occurring as a ternary process, perhaps including ammonia or an organic compound; 2) some other compound in addition to, or instead of, sulfuric acid is participating in binary nucleation; and 3) a heterogeneous process, perhaps involving nucleation of vapor onto ion centers, is occurring. Although none of the above three possibilities can be categorically eliminated, the ternary theory of sulfuric acid/ammonia/water is increasingly viewed as the most likely mechanism.[57] This view is aided by the observation that the presence of ammonia in sulfuric acid/water solutions considerably decreases the vapor pressure of sulfuric acid.[58] In addition, laboratory studies have shown that very low levels of tens of parts per trillion of ammonia can boost nucleation substantial-ly.[59] A heterogeneous process of nucleation on ion centers might also explain current observations of nucleation.[54] Laboratory investigations have shown that the presence of gaseous ions can enhance nucleation rates.[61] Yet, to date, there have been no measurements of the composition of atmospheric ions during nucleation events to validate this hypothesis.
Fig. 9 Comparison of conditions that resulted in nucleation events at various sites in the remote troposphere to predictions of the onset of sulfuric acid/water binary nucleation using two CNT models that include hydrate effects (solid and dashed lines).
In the review of observations mentioned above, observed particle diameter growth rates are typically in the range of 1-26 nm/hr in midlatitudes, and depend heavily on the presence of condensable vapors and temperature. Attempts to interpret these observations by using Eq. 3 for the condensation of sulfuric acid and water generally underpredict growth by a factor of 5-10.[5] Either other species are contributing to particle growth, or growth is occurring by other processes in addition to monomer addition. The former option is quite likely to occur, as many oxidized organic compounds have sufficiently low vapor pressure to contribute to aerosol growth. Unfortunately, the property that makes such compounds candidates for condensational growth also makes them extremely difficult to study. For the latter option, preliminary results from the ANARChE study suggest that growth can be accounted for by including cluster condensation in Eq. 3.
CONCLUSION
Atmospheric nanoparticles, which are defined here as particles with a spherical equivalent diameter smaller than 50 nm, are now understood to be important atmospheric constituents that can dominate a particle number distribution spectrum. They can be directly emitted from combustion sources, but on a global scale, the most important formation process is by homogeneous nucleation. Nanoparticle growth, which in some locales can be as rapid as 26 nm/hr, can occur as a result of coagulation and physical uptake of compounds into the aerosol. Such growth links the presence of nanoparticles to a variety of phenomena such as climate, health, visibility degradation, and chemical transformations in the atmosphere. Measurement techniques are already able to detect and size nanoparticles at sizes as small as 3 nm. Future advances may include the ability to detect postnucleation clusters, which would require detection sensitivity to about 1 nm particles. Chemical composition techniques are undergoing rapid progress, with on-line instruments now capable of characterizing the composition of particles in the 5-20 nm diameter range. Modeling of the formation and growth of nanoparticles has traditionally relied on CNT and approximations to key nanoparticle physical properties such as the capillarity assumption. These have found some success at predicting formation rates in some parts of the atmosphere, but for the majority of locales, theories based on binary CNT are overpredicting the chemical environment required for nucleation by several orders of magnitude. Ternary nucleation of sulfuric acid, ammonia, and water may be the solution to this discrepancy, and is expected to be an important component of future theoretical advances.