INTRODUCTION
Biological molecules such as antibodies show exquisite specificity in binding to target molecules. This binding is naturally reversible, and the stability of the antibody-target complex is often a matter of concern because experiments can be compromised if it dissociates or exchanges with other molecules. Here we describe the current state of molecular technology for making specific complexes irreversible, so that target specificity is preserved while dissociation is effectively prevented. The emphasis is on antibody applications, but the same concepts apply to other binding systems.
Statement of the Problem
One of the most important elementary phenomena in biochemistry and biology is the reversible binding of one molecule to another:
If A is an antibody and B is its target, this can be the first step in resistance to infection; if A is a drug molecule and B is its receptor, this can be the basis for pharmacological action; there are many other fitting examples. An important feature of this process is that binding is specific: sites on molecules A and B fit together to form a complex, but A and B do not bind indiscriminately to other molecules. Specific binding is generally achieved by complementary noncovalent interactions between A and B, such as hydrophobic bonding, hydrogen bonding, ion pairing, and pi-stacking. In biological systems, binding of A and B is usually accompanied by the release of several water molecules from the surface of each. This provides an entropy change in favor of complex formation.
Reversible binding (Eq. 1) is quantitatively characterized by rate constants kj and k2, and an equilibrium constant Kd:
At equilibrium, k1[C] = k2[A][B] and
The brackets [] denote concentrations, in units of mol/L, M. Rate constants kj and k2 have different units: typically, kj is expressed in units of sec"1 for the first-order dissociation process, while k2 is expressed in units of M"1 sec"1 for the second-order (bimolecular) association process. The units of Kd = k1/k2 are then mol/L, M. A common rule of thumb is that useful pharmaceuticals should bind to their targets with dissociation constants Kd< 10"9 M. Note that when [A] equals Kd, then [B] = [C] and the target B is half-saturated (the complex C is formed with 50% yield).
The rate of the association reaction can be no faster than the rate at which A and B diffuse together, and it is usually found that k2 < 109M"1 sec . We can use this to estimate the minimum bound lifetime of the complex. For the case Kd= 10"9 M, at maximum k2= 109 M"1 sec"1, and the maximum value of k1 = 1 sec"1. From fundamental mathematics, the lifetime t of the average complex in Eq. 2 is t = 1/k1, which in this case would be 1 sec. A bound lifetime of 1 sec would be too short for many practical applications. (Because we used a maximum value for k1, we have calculated a minimum value for t. Few experimental on-rates are as large as 109M"1 sec" 1: for a given Kd, if the on-rate k2 is smaller, then the off-rate k1 is also smaller, and the bound lifetime of the complex is longer.)
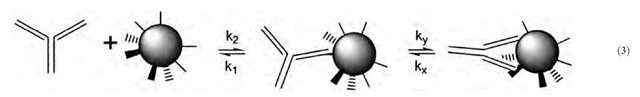
Biological molecules can exhibit more complicated properties than those described above. One common variation is that macromolecules may incorporate more than one binding site for target molecules, and undergo multivalent binding.
ADDED COMPLICATIONS OF MULTIVALENT BINDING
Elementary Kinetic Scheme has System-Dependent Terms
A ligand-receptor system in which the binding equilibrium can be more than monovalent is illustrated by the divalent antibody molecule binding to the polyvalent target in Eq. 3. Association of the antibody to its target occurs in two distinct steps: first, the familiar association from two separate molecules with rate constant k2, and second, a unimolecular ring-closing reaction with rate constant ky sec~1. The latter reaction involves the same ligand-receptor interaction chemistry as the bimolecular association, but here the process does not require two separate molecules to diffuse together.
It should be noted that the equilibrium constant for bivalent association is not calculated simply by squaring the equilibrium constant for monovalent association. It is commonly found for antibody binding to multivalent targets that bivalent association decreases the overall dissociation constant by a factor of 100-1000 relative to monovalent association, making binding more stable but not vastly so. However, in some cases, such as cancer cells that overexpress a particular cell-surface protein, a bivalent antibody can associate with an antigen-rich surface practically irreversibly. This has inspired considerable effort in developing systems that bind multivalently to enhance the strength of the binding interaction in practical situations. A common example is the engineering of antibodies and other proteins for multivalent binding to carbohydrates, for which monovalent binding tends to be weak.
Effective Local Concentration
With reference to Eq. 3, it is possible to take the ratio of ky/k2, which is expressed in mol/L, as indicating the effective local concentration of the target in the vicinity of the monovalently bound antibody molecule. When experimental results are available, this provides an index of the density of targets on the particle in Eq. 3 and their accessibility to the second arm of the antibody molecule— a composite quantity that is difficult to calculate. The effective local concentration is sometimes a nonphysically large quantity, indicating favorable geometric and entro-pic properties for forming the second association.
Multivalent binding can have unwanted consequences: for example, when an antibody binds two targets on a cell surface, it can trigger the internalization of the complex and subsequent changes in the cell’s behavior. Of course, if the targets are not linked together, binding of an antibody to two targets involves two independent bi-molecular association events as in Eq. 1, which does not increase binding strength. For these reasons, it is frequently desirable to enhance the strength of monova-lent binding.
From the protein engineering viewpoint, reversible binding may be strengthened by combinatorially varying the amino-acid residues in the binding site, to seek mutant proteins that exhibit thermodynamically more favorable binding.[1] There is considerable activity in developing new experimental approaches to combinatorial selection, and remarkably high binding affinities have been achieved in some cases.[2] Extrapolating this to its logical extreme leads to binding that is so strong it is not reversible.
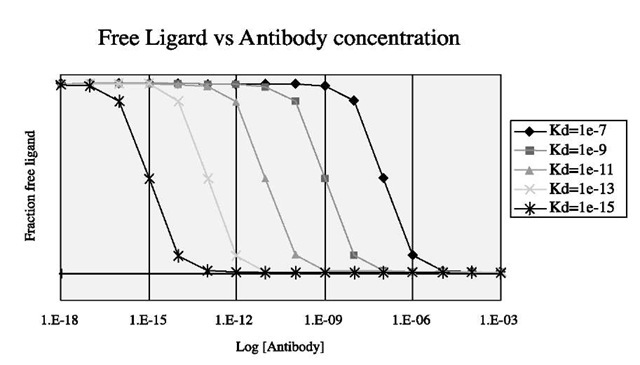
Fig. 1 Binding curves for ligand-receptor systems with different affinities. The horizontal line at the bottom of the graph corresponds to infinite affinity.
Fig. 1 illustrates the progressive effects of increasing binding affinity (decreasing Kd). With reference to Eqs. 1 and 2 above, this shows a semilogarithmic plot of
I as a function of [A] for various values of Kd. Moving from right to left, we see the curves for progressively stronger binding, with their half-saturation points at [A] = Kd. The system with Kd= 1 x 10"7 M has the lowest affinity, comparable to a weakly binding antibody; the system with the highest affinity, Kd = 1×10"15 M, is comparable to the strongest-binding systems found in nature. Reducing the concentration of free target to 50% requires an amount of free antibody [A] = Kd; reducing the concentration of free target to 1% requires an amount of free antibody [A] = 99Kd; reducing the concentration of free target to 0.1% requires an amount of free antibody [A] = 999Kd; and so on.
Infinite binding affinity corresponds to Kd = 0, which is the horizontal line at the bottom of Fig. 1. Here binding is irreversible and a 1:1 ratio of antibody to target is sufficient to reduce the concentration of free target to zero at equilibrium; no excess is required. Obviously, there will be practical limitations on literally achieving a zero concentration of free target with a precisely 1:1 ratio of antibody to target, because as the concentrations of antibody and target become increasingly small, the rate of the second-order association reaction (= k2[A][B]) tends to zero. However, a small excess of antibody can make a difference at this stage: as target B becomes depleted, the concentration [A] of antibody becomes much larger than [B] and, as a consequence, ceases to decline significantly. The experimenter can control the rate of the association reaction (still = k2[A][B]) by choosing the amount of A to add to the system.
PRINCIPLES OF IRREVERSIBLE BINDING
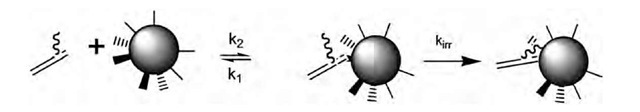
Consider an engineered monovalent antibody (fragment) that can bind irreversibly to its target. The molecular mechanism of this process is illustrated in Eq. 4, consisting of a reversible binding step followed by an irreversible locking step. The mathematics describing irreversible binding are not unlike those for bivalent binding (Eq. 3). Conceptually, molecular recognition of the target and reversible binding is the first step in both; it is followed by an intramolecular ring closure that involves either a second binding site (Eq. 3) or a reactive moiety in or near the first binding site (Eq. 4).
The difference is that we replace ky with kirr and kx = 0 for Eq. 4. The most valuable situation for irreversible pharmacological targeting is when kirr ^ kj, so that the complex is formed irreversibly upon binding, without dissociation. Irreversible binding with little or no dissociation has been achieved for an antibody that binds a small molecule, using synthetic chemistry to engineer the small molecule and site-directed mutagenesis to engineer the antibody.[3] This is referred to as infinite affinity, because the off-rate is effectively zero. Because the locking reaction must occur within the lifetime of the complex t = 1/kj, infinite affinity is easier to achieve when k1 is small.
EXPERIMENTAL BACKGROUND
In the past, enzymologists, immunologists, and other professionals prepared reactive organic molecules that resembled known enzyme inhibitors or antibody haptens, and identified important residues in the respective protein binding sites by forming stable covalent bonds and analyzing the products. The reagents have employed relatively reactive electrophiles such as isothiocyanates, sulfonyl fluorides, mustards, or nitroaryl halides.[4-7] Under appropriate conditions, these electrophiles exist for a useful period of time in aqueous solution, although they are quite reactive. When the electrophilic inhibitors are bound to their targets, the effective local concentration of protein nucleophiles (particularly thiols, but also amino groups, etc.) near the bound electrophile can lead to specific attachment to one or a few amino acid side chains.
The concepts underlying these in vitro experiments can be extended to developing molecules for use in vivo, by developing ligands that are unreactive to the many nucleophiles that occur in living systems but nonetheless react efficiently with their specific protein molecules. When the protein has a conveniently located cysteine sidechain, this can be accomplished by judicious chemical synthesis of the ligand. In some other circumstances, a cysteine can be engineered into the protein.
The favorable situation of a uniquely reactive group advantageously placed in a binding site is not often found in nature. An exceptional example from the pharmaceutical literature is given by Fry et al.[8] who synthesized 4-anilinoquinazolines carrying mildly electrophilic acryl groups, which proved to bind irreversibly to one of the cysteine residues in the catalytic domain of the epidermal growth factor receptor (EGFR). Another work with a,p-unsaturated carbonyl compounds as enzyme inhibitors further supports this approach to irreversibly blocking an important binding site by Michael addition to a cysteine side chain.[9] When it is part of a rapidly cleared small molecule, the a,p-unsaturated carbonyl compound does not detectably react with nucleophiles normally present in the blood because the contact time is short and they are present in low concentration.[10] The most important nucleophiles in circulation are thiols on glutathione and other small molecules, and the free cysteine side chain in albumin.
ENGINEERING INFINITE AFFINITY
Converting a natural protein to irreversible binding has been achieved in a few instances, by engineering a cysteine residue into a chosen position in the binding site.[3,11,12] Here the aim is to exploit the exceptional nucleophilicity of a well-placed sulfur atom to form a stable covalent bond. In applications reported so far, the other binding partner has been a small organic molecule. Thus a double engineering approach is used, relying on the tools of modern molecular biology to design and produce a mutant protein, and synthetic chemistry to produce the small molecules that target the engineered binding site on the protein.
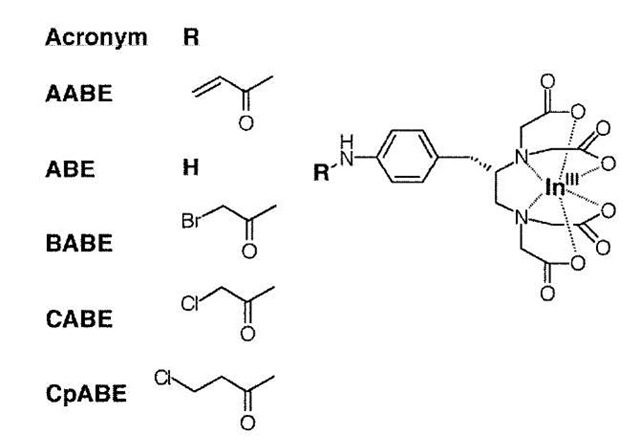
Fig. 2 Crystal structure of antibody CHA255-indium chelate complex (From Ref. [13].) Two residues in the wild-type antibody that are not directly involved in ligand binding but are favorably located close to the para-substituent of the ligand (yellow) are light-chain residues S95 and N96 (Kabat positions 93 and 94).
Fig. 3 Electrophiles tested for: 1) lack of reactivity in complex physiological media; and 2) specific reaction with engineered antibody S95C (Fig. 2).
Planning the experimental strategy is made easier if a crystal structure of a natural complex is available. Chmura et al.[3] referred to the crystal structure of the antibody CHA255 bound to its indium-chelate hapten.[13] Levitsky et al.[11] used the structure of the cyclophilin A-cyslos-porin A complex.[14] In each case, amino-acid residues that were near, but not in contact with, the bound organic ligand were identified.
Chmura chose residues within the binding site of the antibody (Fig. 2). These residues, which are in complementarity determining region 3 of the light chain, were chosen because their side chains: 1) are not exposed on the outer surface of the antibody; 2) do not have any direct contacts with the bound ligand; and 3) lie within a few angstroms of the para substituent on a benzene ring in the ligand. The latter criterion allowed for ease of synthesis of a library of candidate ligands for irreversible binding. On the other hand, Levitsky chose protein residues that: 1) are exposed on the outer surface of the protein; 2) do not have any direct contacts with the bound ligand; and 3) lie within a few angstroms of a suitable site for synthetic modification of the ligand. In each case, site-directed mutagenesis was used to prepare cysteine mutants in the chosen positions. The proteins were expressed by using appropriate cell lines, and characterized by standard methods.
Chmura compared a set of electrophilic substituents (Fig. 3), not only for specific reaction with the engineered cysteine side chain but also for physiological clearance in animal models, settling on the acryl group.[10] This agrees with the experience of other researchers who have sought to use the Michael addition to cysteine sulfur for permanent binding under physiological conditions.
The attack of a sulfhydryl group on the double bond of a nearby a,p unsaturated carbonyl compound appears to have favorable properties for use in site-directed protein targeting under physiological conditions in vitro and in vivo. Under these conditions, the reaction is irreversible. The attachment is stable even to the conditions of boiling and reduction that accompany preparation of a sample for gel electrophoresis.
CONCLUSION
Infinite affinity binding systems are at a very early stage. However, it seems clear that they offer unique advantages in practical applications where irreversible association is needed. A potentially fertile area is in pharmaceutical applications where elimination of a specific molecule is required. For example, antibodies against growth factors are under development as cancer therapies.[15] It may prove worthwhile to completely eliminate a particular growth factor by irreversible binding. In the area of in vitro assays, the avidin-biotin binding pair is widely used in circumstances where practically irreversible binding, e.g., of a biotinylated protein to a surface, is required. Avidin only binds biotin, and the binding is not actually irreversible. Many systems with infinite affinity are possible, offering the possibility of multiplexed sets of orthogonal binding pairs to localize different ligands permanently and specifically on the same surface. On the other hand, for those applications that require buffering the concentration of a ligand within a particular concentration range, reversible binding is intrinsically superior as long as a binding partner with an appropriate Kd is available.


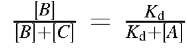
![Crystal structure of antibody CHA255-indium chelate complex (From Ref. [13].) Two residues in the wild-type antibody that are not directly involved in ligand binding but are favorably located close to the para-substituent of the ligand (yellow) are light-chain residues S95 and N96 (Kabat positions 93 and 94). Crystal structure of antibody CHA255-indium chelate complex (From Ref. [13].) Two residues in the wild-type antibody that are not directly involved in ligand binding but are favorably located close to the para-substituent of the ligand (yellow) are light-chain residues S95 and N96 (Kabat positions 93 and 94).](http://what-when-how.com/wp-content/uploads/2011/03/tmpD5183_thumb_thumb.jpg)