Silver
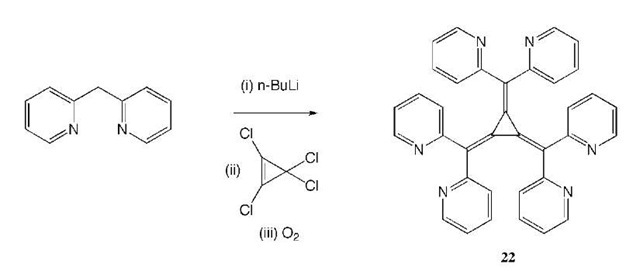
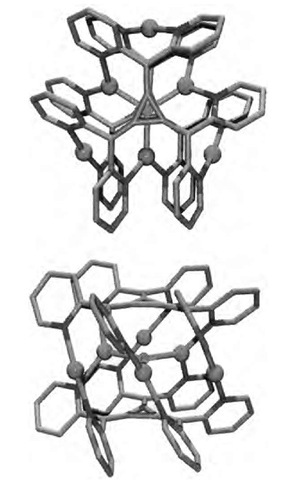
Steel and Sumby have recently reported the formation of cages and polymers with silver as the metallic com-ponent.[29,30] Using hexa(2-pyridyl)[3]radialene (22), prepared from reaction of diarylmethyl anions with tet-rachlorocyclopropene[31,32] and reaction of this with silver tetrafluoroborate produces [Ag(222)F(BF4)5-11H2O], a hexametallic cage, with each 22 acting in a hexapodalhexadentate mode (Scheme 4). Within the cavity is a fluoride ion bound in a trigonal arrangement to three silver atoms with the other three arranged symmetrically away from this (Fig. 12). Reaction of this same ligand with silver nitrate yields a coordination polymer with each ligand coordinated to four silver atoms, in a twisted helical arrangement.
Scheme 4 Route to the hexanuclear silver array.
Fig. 12 Two perspective views of the Ag6(22)2F.
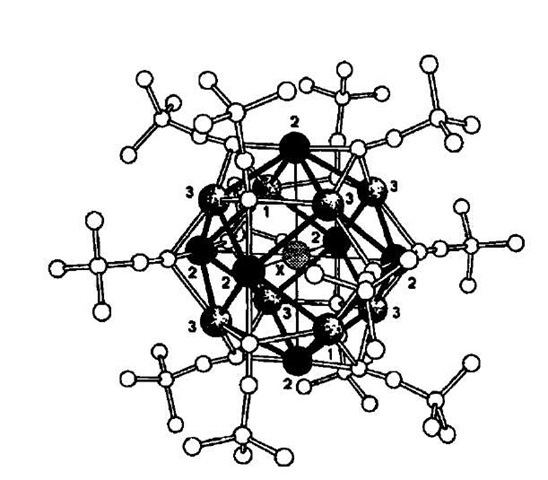
Further examples of the use of silver are provided by Rais and coworkers. These researchers have reported the synthesis of [Ag14(C=CfBu)12Cl]OH, from a solution of AgBF4 being mixed with fBuC=CH and NEt3 to form the initially insoluble polymeric [Ag(C=CfBu)]„ which upon the addition of chloride is transformed into the metallac-age 23 (Fig. 13).[33] More recently, the more direct route from AgBF4, ‘BuC=CH, NEt3, and NMe4Cl has been developed.[34] Via this same route [Ag14(C=CfBu)12X] [BF4] (X=F, Cl, Br) has also been prepared. Each cage has a single halide at its center and is formed through halide templation and metallophilic interactions (Fig. 14). The use of the alternative silver salts AgTos and AgNO3 produced only polymeric compounds (Tos= p-toluen-sulfonate).
Fig. 13 Structure of the silver cage complex 23.
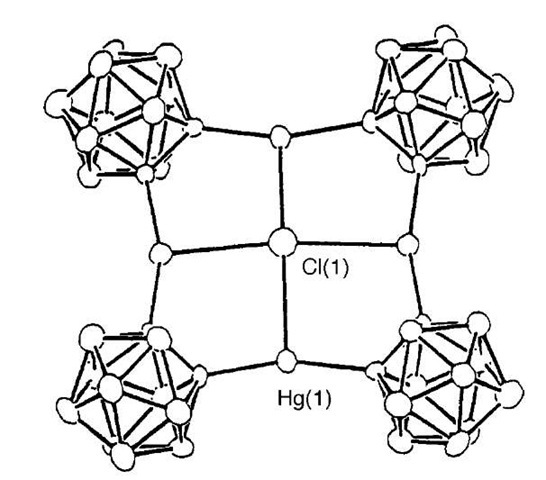
Fig. 14 X-ray crystal structure of the chloride complex of 25-CT.
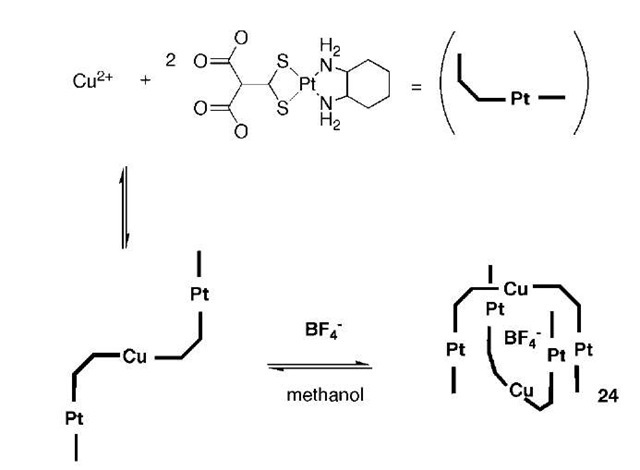
The first example of an inorganic ”tennis ball” motif encapsulating an anion has been reported by Kim and co-workers.[35] Dissolution of the complex {(dach)Pt(BET-MP)}2-Cu(BF4)2 (dach = trans( ± )-1,2-diaminocyclo-hexane, BETMP=Bis(ethylthio)methylenepropanedioate) resulted in the formation of complex 24 shown in Scheme 5. The starting material contains hydrogen bond acceptors (carboxylate) and donor (amine) groups, allowing the complex to dimerize around a central BF4 anion giving the tennis ball motif.
One of the first examples of work in this area is the mercuracarborand macrocycles reported by Hawthorne et al.[36-38] Preparation of tetraphenyl [12]mercuracarborand-4 (25) was achieved via the reaction of mercuric chloride with 1,2-dilithiocarborane (Scheme 6). Addition of mercury chloride templated the lithiated carbo-ranes into a tetrameric cycle around a chloride template (Fig. 14). Synthesis of the iodide analogue was successfully performed, but instead of the anion being incorporated into the plane of the cycle, it was perched 1.25 A above the plane. Use of nonspherical anions resulted in the formation of acyclic compounds instead of the desired macrocycles.
Scheme 5 A schematic representation of the arrangement of the platinum, copper, and BF4 components.
Scheme 6 Synthesis of [12]mercuracarborand-4 25.
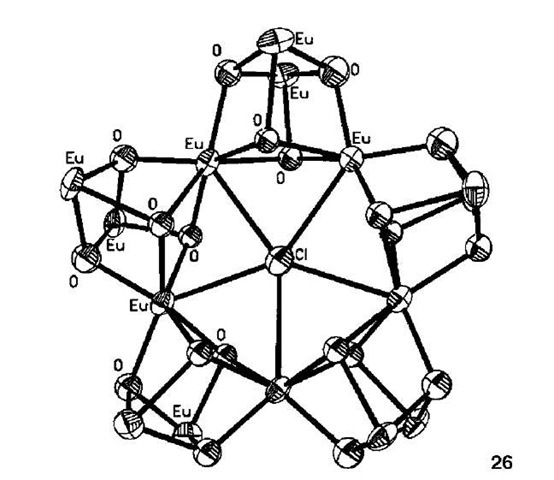
Very few examples of lanthanide clusters templated by anions have been reported. The exception is the series of lanthanide (III)-tyrosine metallamacrocycles synthesized by Wang and coworkers.[39,40] The clusters were prepared by L-tyrosine-controlled hydrolysis of the appropriate lanthanide perchlorate, where Ln=La, Pr, Nd, Sm, Eu, Gd, and Dy. In all cases an isostructural pentanuclear structure resulted. Below, as an example, is the europium (III)-tyrosine metallamacrocycle 26 (Fig. 15), with core formula [Eu!5(m3-OH)2o(m5-X)]24+.
Its pentameric structure is clearly demonstrated with the Eu ions forming three layers, each containing five ions. At the center is a chloride ion, which simultaneously coordinates the five inner-layer Eum atoms. The formation of this complex can best be described as a chloride-induced self-assembly, i.e., the chloride is acting as a template. When a lanthanide perbromate was used the equivalent pentameric complex with a bromate at the center was produced. Yet when iodide was used as the templating agent an alternative dodecanuclear lanthanide complex was formed, with a core formula of [Ln12(m3-OH)16(I)2]18+ (Ln=Dy, Er). Such complexes have a square cyclic arrangement with an iodide on either side of the square plane. Syntheses that do not use halides as the templating agent do not produce these penta and dodecanuclear species.
Fig. 15 The europium-based cubane structure.
Fig. 16 The X-ray crystal structure of complex 30.
Scheme 7 Preparation of both the tetra and pentameric forms of ![]()
Main Group
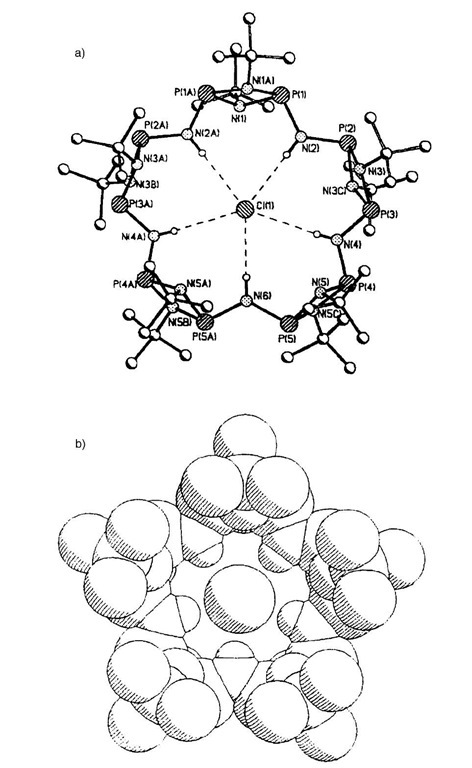
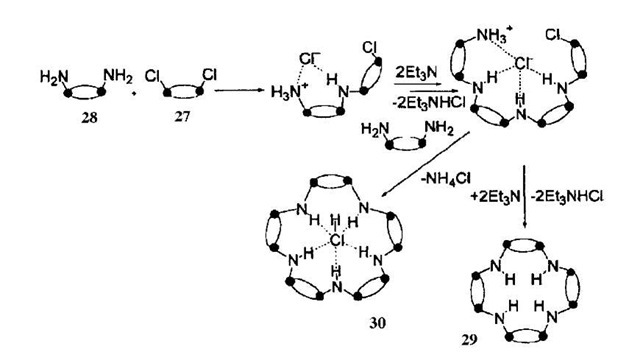
Wright and coworkers have investigated the formation of a series of macrocycles, from the reaction of [ClP(m-N'Bu)]2 (27) with [NH2P(m-N'Bu)]2 (28) in the presence of a base to produce a {[P(m-N'Bu)2](m-NH)}2n frame-work.[41] In the absence of a templating anion, the tetrameric species 29 is formed (i.e., n=4), but when the same reaction is performed in the presence of an excess of LiCl2 then the major product is the pentameric form [{P(m-N'Bu)2}(m-NH)]5(HCl) 30 (Fig. 16; Scheme 7). The templating chloride ion is found at the center of the macrocycle, held in position by five hydrogen bond interactions between it and the NH groups.
CONCLUSION
We believe that the recent advances in the use of anions in self-assembly processes will lead to their employment in the templation of both inorganic and organic supramolec-ular nanoarchitectures in the future. This area of chemistry is yet to be fully explored and in particular the exploitation of the geometry of the anionic guest to guide the assembly process has untapped potential for the construction of new supramolecular species. We look forward to advances in this area of chemistry in years to come.
![Scheme 6 Synthesis of [12]mercuracarborand-4 25. Scheme 6 Synthesis of [12]mercuracarborand-4 25.](http://what-when-how.com/wp-content/uploads/2011/03/tmpD5143_thumb_thumb.jpg)