INTRODUCTION
The use of anions to template the formation of new supramolecular entities is a relatively new approach to self-assembly, but one that is expanding the available number of noncovalent and coordinate bonding motifs for the construction of new interlocked materials and new supramolecular nanoarchitectures.[1-5] Transition metal-directed approaches (e.g., using a transition metal to template catenane formation) rely on the directionality of the coordination sphere of the metal to define the geometry of the new self-assembled entity. Hydrogen bonds to anions are also directional in nature,[6] and this, combined with the range of geometries that anions possess, allows them to be used to template a variety of new organic supramolecular species. The range of anion geometries has also allowed anionic species to be employed in templating roles in the formation of inorganic clusters and new materials.
This chapter focuses on ”inorganic” systems, subdivided by the type of metal cation used. Readers interested in organic systems are directed to the article on ”Anion-Templated Self Assembly: Organic Compounds,” keeping in mind that there are many overlaps between these categories and this division is intended only as an aid to the reader.
INORGANIC ANION-TEMPLATED SYSTEMS
Vanadium
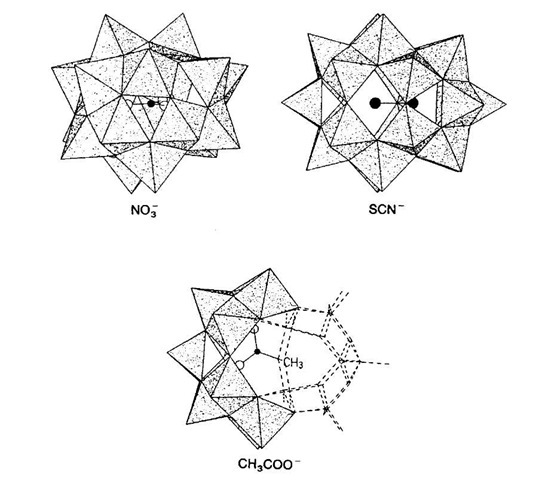
Muller and coworkers have extensively investigated the roles anions can play in templating the formation of structures from linked Vn+Ox polyhedra.[7] Specifically, Muller and coworkers have shown that anionic templates such as nitrate and acetate can control the linking of these species.[8,9] For example, different shell-like clusters were shown to form by reduction of vanadate (V) or oxidation of vanadate (IV)/vanadate (V) species in water in the presence of anions such as NO^ (1), SCN~ (2), and CH3COO- (3) (Fig. 1).
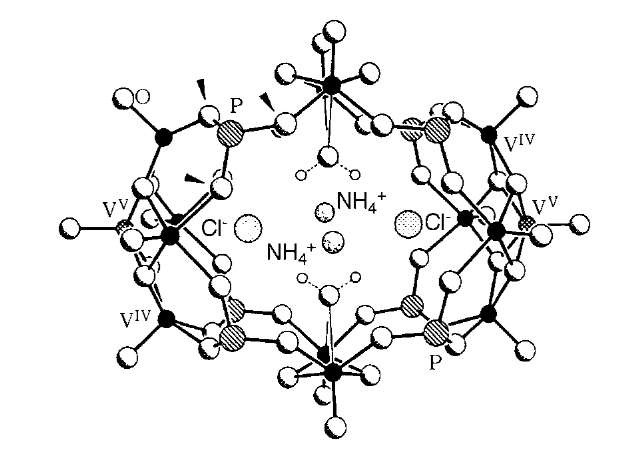
Oxo-vanadium species can be templated by ion-pairs.[10] In the central cavity of the system [V14O22- (OH)4(H2O)2-(C6H5PO3)8]6 " (4), Muller and coworkers have shown that two chloride ions and two ammonium ions reside, the templation of this cluster being reliant upon the presence of the ion-pair (Fig. 2).
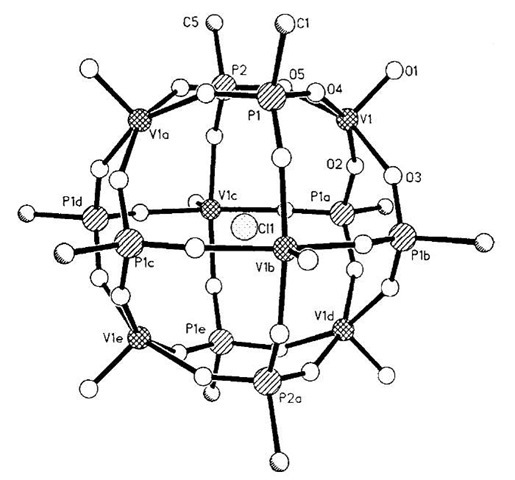
Salta and coworkers have also shown the templating ability of chloride ions to assist the formation of oxovanadium-organophosphate cluster structures. They have synthesized a number of cage structures containing differing numbers of templating chlorides. For example, cluster 5 shown in Fig. 3 contains a single chloride template (Fig. 3).[11]
Iron
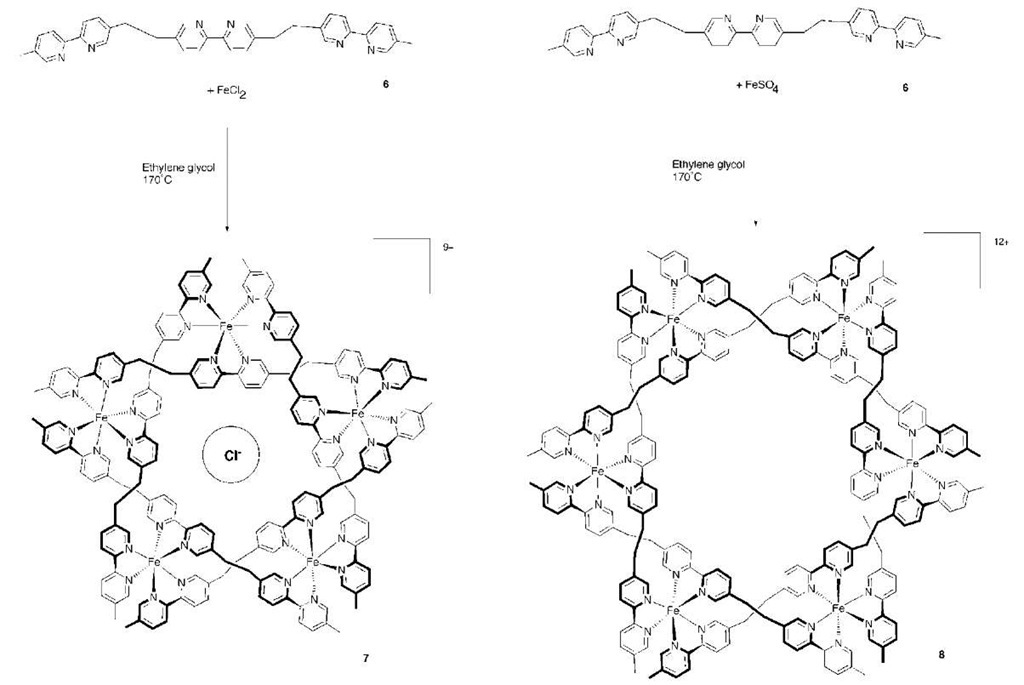
Hasenknopf and coworkers have used iron bipyridyl complexes to form a pentameric circular helicate around a chloride anion. The formation of this circular species was found to be dependent on the nature of the iron salt present. Complexation of iron chloride by the tris(bipyr-idine) ligand (6) produced the pentameric structure [Fe5L5Cl]9+ (7), templated around a chloride ion locked at the center of the structure (Scheme 1). Attempts to use bromide as the templating anion produced a mixture of the penta- and hexameric structures, while the use of nonhalide iron salts, such as iron sulfate or tetrafluorobo-rate, produced only the untemplated hexameric structure 8.[12,13] Further work revealed that the above reaction of FeCl2 (and also the analogous one using nickel) progressed first through a linear helicate, which then progressively transformed into the circular structures.1-14-1 Use of the longer ligand produced a tetrameric double helicate, irrespective of the type of iron salt used.
Cobalt
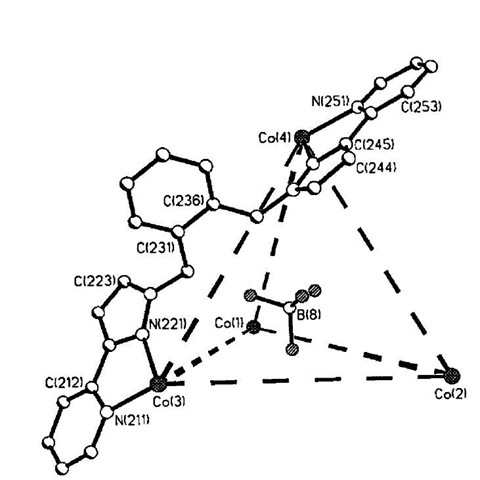
Cobalt-based tetrahedral supramolecular arrays have been reported by Fleming and coworkers.[15] Cage complexes have been synthesized from a pyrazoyl-pyridine ligand by reaction of the ligand with the appropriate metal acetate hydrate in methanol followed by addition of aqueous sodium tetrafluoroborate (resulting in the complex precipitating from solution). As shown in Fig. 4, a BF4 anion is located at the center of the tetrahedral cage[9] formed by the four cobalt(II) metal ions at the vertices and six bridging ligands, thus each cobalt is octahedrally com-plexed. Each of the fluorines of the encapsulated anion is directed toward the triangular faces of the cluster rather than the metal ions, implying electrostatic interactions are the driving force for this templation process, the anion partially balancing the 8+ charge of the ring. Presumably, the anion has adopted an orientation dictated by the geometry of the internal cavity. Multiple p-stacking interactions between the ligands also assist the assembly of the structure. Such a configuration can also be templated by QO4, but in the absence of any template anion the cage does not form. Interestingly when nickel is used a different structure is formed (a dinuclear structure species with no templating anion). If an analogous ligand containing a biphenyl spacer is used, then a larger tetrahedral complex forms; however, the central cavity is empty, i.e., the system is not templated by an anion.[16] In solution it has been shown that the BF4 ions diffuse in and out of this cavity. This example illustrates how delicately balanced these systems are, and how changing only one aspect of the system in a small way can have a dramatic impact on the assembled structure.
Fig. 1 Anion-templated polyhedral V-O structures 1 (top left), 2 (top right) and 3 (below).
Fig. 2 Structure of the oxo-vanadium cluster 4 showing templating ammonium and chloride ion-pairs in the center of the cavity.
Fig. 3 A view of the structure of 5 showing chloride template.
Scheme 1 Chloride-templated assembly of a pentameric circular helicate.
Fig. 4 Part view of the tetrahedral cage structure 9, with the central templating BF4 anion.
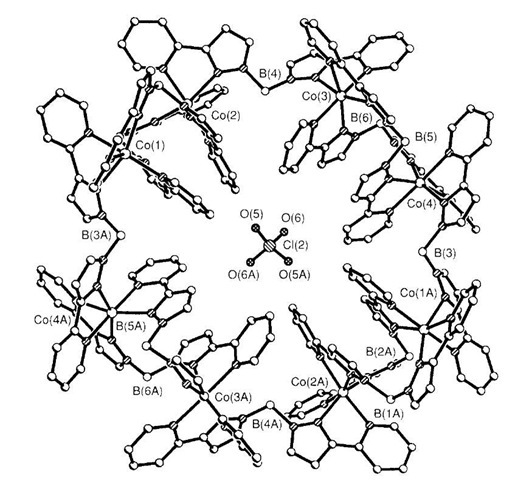
The same research group have used cobalt in combination with abis{3-(2-pyridyl)pyrazol-1-yl}dihydroborate ligand to form a cyclic structure encapsulating a perchlo-rate anion.[17] Reaction of [bis{3-(2-pyridyl)pyrazol-1-yl}dihydroborate] with cobalt(II) produced the cycle 10 shown in Fig. 5. Each ligand acts as a bridge between two adjacent metal ions, with an alternating pattern of one and then two ligands. The isostructural octanuclear ring is also formed if Ni(II) is used.
Nickel
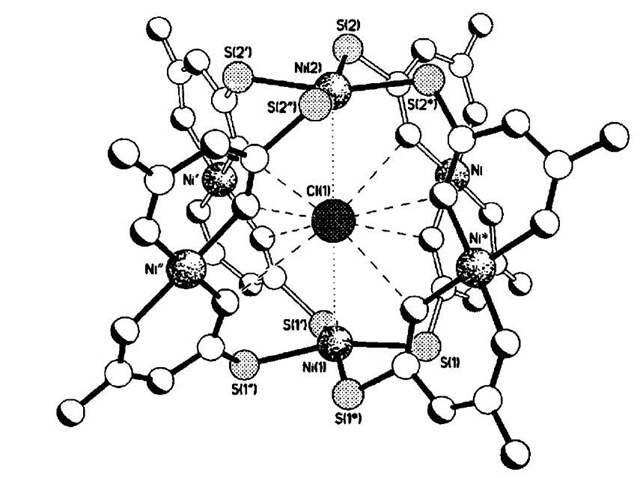
Mingos and coworkers have synthesized metallamacro-cycles and metallacages, by reacting NiCl2 with atu (Hatu = amidinothiourea) to produce the monomer [Ni(atu)2], with further reaction yielding the metallacage [Ni6(atu)8Cl]Cl3 (11).[18] The eight amidinothiourea units coordinate to the six nickel ions through the nitrogen and sulfur atoms via hydrogen bond interactions (Fig. 6). A single chloride ion was identified as being bound at the center of the cage, through eight N-H-Cl hydrogen bonds and Lewis acid/base interactions utilizing the two NiS4 units. The analogous bromide species [Ni6-(atu)8Br]Br3 has been synthesized. Confirmation of the templating role of the halide anion was provided by the failure of nitrate, acetate, or perchlorate to produce the desired cage, and instead monomer salts [Ni(Hatu)2]2+ were formed. However, subsequent addition of halide ions to the salt was found to generate the cage complex.
Fig. 5 Crystal structure of the cobalt cage 10 with the central ClO4 ion.
Fig. 6 Structure of the nickel cage 11 showing the central chloride
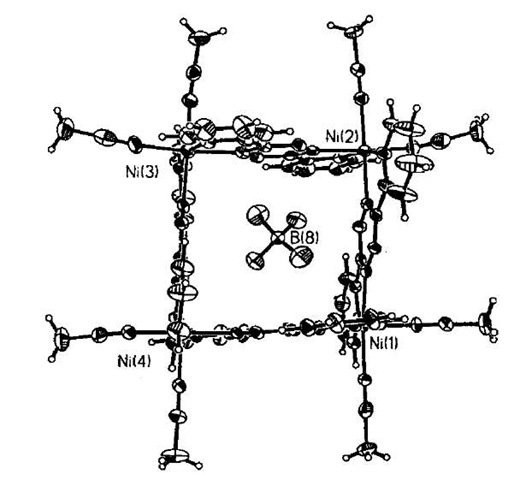
Fig. 7 Dunbar’s tetrafluoroborate templated nickel square 12.
Campos-Fernandez and coworkers have also used nickel in anion-templated complex formation.[19] When solutions of [Ni(CH3CN)6][BF4]2 and bptz (3,6-bis(2-pyridyl)-1,2,4,5-tetrazine) were mixed in a 1:1 molar ratio the molecular square [Ni4(bptz)4(CH3CN)8][BF4]8 (12) was produced (Fig. 7). One BF4 anion is held in the internal cavity of the square. A similar assembly is also produced when zinc is used instead of nickel, and in both these cases the templating ion can be either the tetra-fluoroborate or perchlorate. However, if the larger anion SbFg is used (by starting from [Ni(CH3CN)6][SbF6]), a pentameric compound is obtained as the major product [Ni5(bptz)5(CH3CN)10] [SbF6]10.[20]
Fig. 8 The hexa-molybdenum-based complex 13.
Molybdenum
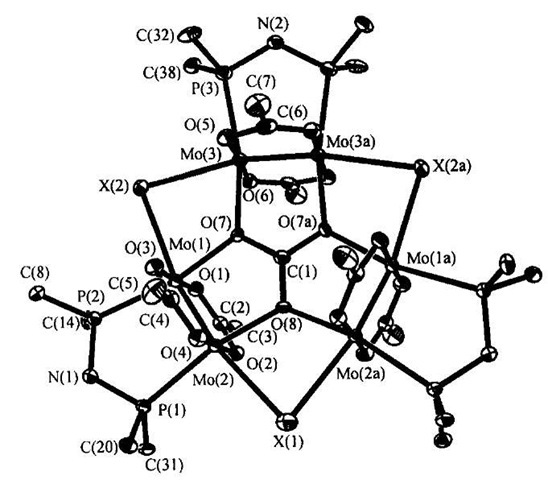
A number of supramolecular structures using second-row transition metals have been reported. Chen and coworkers have employed metal-metal bonded molybdenum species to form the cyclic hexanuclear structure {[trans-Mo2(O2CCF3)2(m-dppa)]3(m-CO3)(m-X)3F} (X=Cl, Br, I) (13). This complex is the product of the reaction between [trans-Mo2(O2CCF3)2(MeCN)6][BF4]2, K2CO3 and dppa (N,N’-bis (diphenylphosphine)amine).[21] The quadruply bonded Mo2 units are held in position by the central carbonate ion. It is assumed that the chloride ions present in the complex originate from the solvent as the analogous Br and I complexes are formed during the reaction in acetonitrile and the subsequent addition of ZnX2 (X=Br or I) (Fig. 8).
Palladium and Platinum
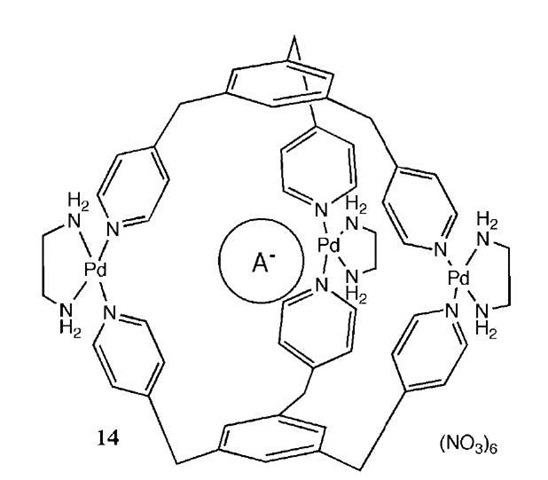
Fujita and coworkers have recently reported the synthesis of palladium(II) cage complexes around hydrophobic anions.[22] [enPd(NO3)2] was added to the tridentate ligand 1,3,5-tris(4-pyridylmethylbenzene) in the presence of sodium 4-methoxphenylacetate producing the cage structure 14 shown in Fig. 9. A variety of carboxylate salts containing phenyl, naphtyl, or adamantyl groups were used as templates. NMR studies of the complexes suggest that the hydrophobic part of the anion is located within the cavity of the cage while the polar group protrudes from the structure. Neutral linear ligands such as p-xylene were also able to template these systems, but in the absence of a templating anion no cage was produced.
Fig. 9 Anion templated palladium cage 14.
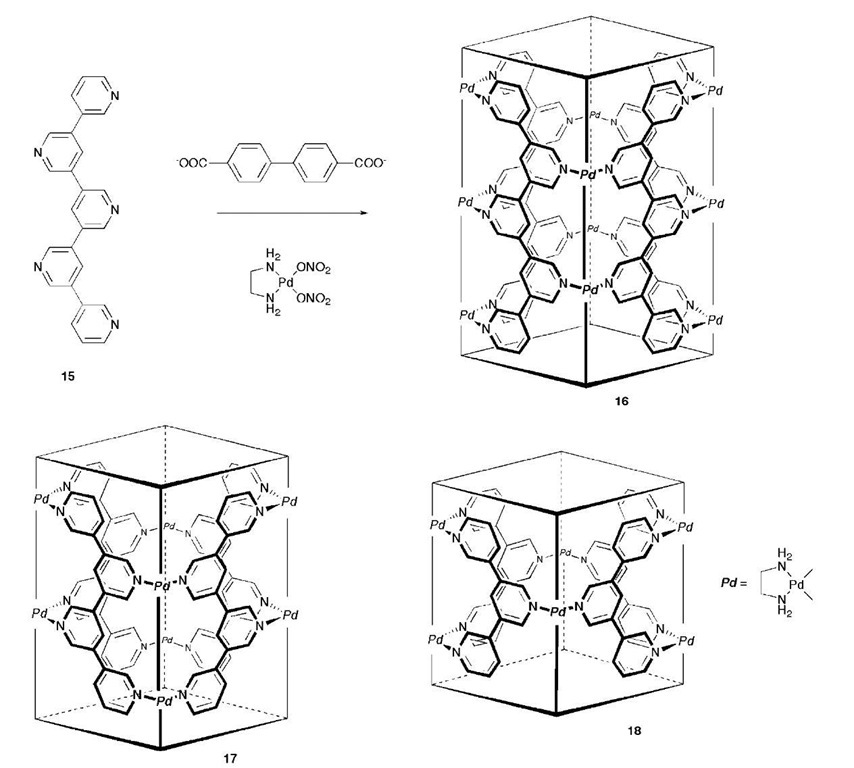
Scheme 2 Formation of the nanotubes 16-18 from pentakis(3,5-pyridine).
More recently, the formation of anion-templated nanotubes has been reported.[23] Reaction of [Pd(en)2-(NO3)2] with pentakis(3,5-pyridine) 15 in the presence of a rod-like anionic template, e.g., 4,4′-biphenylenedicar-boxylate, quantitatively yielded the coordination channels 16-18 (Scheme 2). The reaction is reversible, with the extraction of the template possible at high temperatures. This results in the destruction of the nanotubes, which reassemble upon the re-introduction of the template molecule. Elucidation of the crystal structures of both the cages and nanotubes revealed the linear templates to be bound via combinations of p-p stacking, CH-p interactions, and hydrophobic effects.
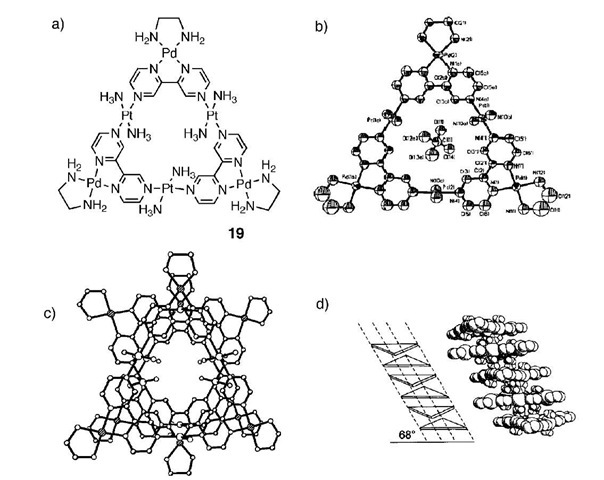
Lippert and coworkers have reported a synthetic solid-state ion channel which incorporates both palladium and platinum.[24] The reaction of [Pt(en)(H2O2)]2+ and 2,2′-bipyrazine (bpz), and subsequent reaction with [Pd(en)]2+ afforded the hexametallic molecular triangle 19 with one of the palladium corner sites displaying a 50% occupancy: [{(en)Pd}2.5(2,2'-bpz)3{NH3}2Pt]3][ClO4]6(NO3).5H2O (en=ethylenediamine, bpz=2.2′-bipyrazine) (Fig. 10a). The molecular triangle hosts within its center one ClO4 ion (Fig. 10b), and in the solid state uses NO4 ions and H2O molecules sandwiched between adjacent triangles to ”glue” the structure together (Fig. 10c and d), the glue in part being a balancing of the cationic charge on each triangle. Thus this structure is templated by the ClO4 anion at an individual unit level and at the macromolec-ular level is held together by NO^ ions and the water molecules. The similar structure [{(en)Pt(bpz)Pd(en)}3]-(NO3)4(PF6)8 was formed by the addition of chelating metal entities such as (en)Pdn to [{(en)Pt(bpz)}3](NO3)6. It has been demonstrated that this molecule simultaneously encapsulates a NO^ ion and a PFg ion at its center.[25] In solution the host demonstrates an affinity for anions such as PFg, ClO4, BF4, and SO2".
Fig. 10 Crystal structure of the palladium-based molecular triangle, and schematic representation of its stacking in solid state.
Fig. 11 The molecular structures of the mixed metal nickel and palladium cages
Scheme 3 Route of formation of the palladium cage, and a schematic showing the association of two of the fluorines with the metal centers.
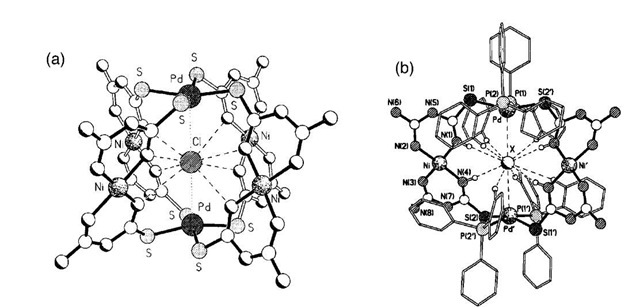
Mingos and coworkers have used palladium in conjunction with nickel to generate mixed metal systems similar to compound 11 above. Adding a solution of [Pd(PhCN)2Cl2] to the nickel monomer Ni(atu)2 produced the mixed metal cage [Ni4Pd2(atu)8X]X3(Fig. 11a).[26] Once again a halide ion is encapsulated at the center of the cage. Either chloride or bromide may be used as the template. The metallamacrocycle [Pd2Ni2(atu)4(PPh3)4-Cl][ClO4]2 was formed via a very similar route: reaction of one equivalent of trans Pd(Ph3)2Cl2 with one equivalent of Ni(atu)2.[27] Two of the Ni(atu)2 groups have been replaced by the four inward pointing PPh3 ligands in the solid-state structure. Once again a chloride ion was found to have templated the formation of the cage by being at the center of the metallacycle (Fig. 11b). The bromide analogue displays the same characteristics; however, in the absence of a halide, monomeric units are produced.
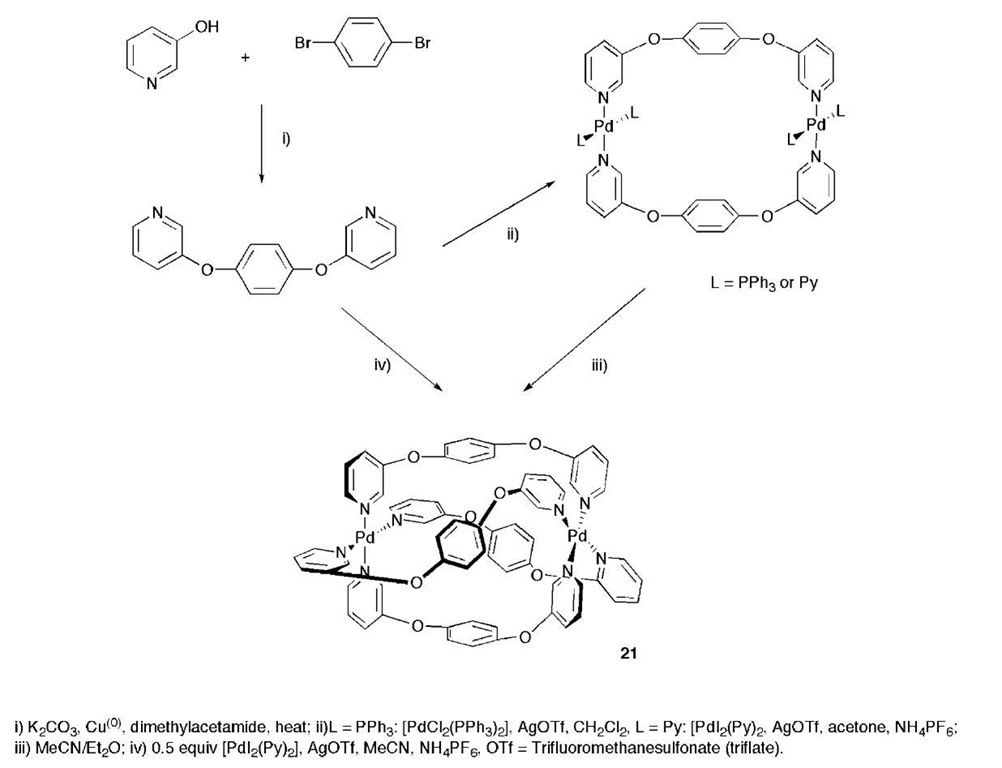
McMorran and Steel have investigated the treatment of the ligand 1,4-bis(3-pyridyloxy)benzene with [PdCl2-(PPh3)2] and [PdI(py)2] in the presence of silver triflate producing dimeric complexes.[28] Crystallization of the dimeric species in the presence of ammonium hexafluo-rophosphate resulted in a reorganization of components to give a M2L4 helical cage 21 as shown in Scheme 3, at the center of which was a PFg anion. The anion forms weak F–Pd interactions, bridging between the two metals in the helical structure.