INTRODUCTION
Research on polymer nanocomposites based on layered silicates has exploded over the last decade,[1-5] ever since the pioneering work of the organoclay-nylon nanocompo-site by Toyota.[6] The dispersion of the silicate nanolayer with its high aspect ratio, large surface area, and high stiffness within a polymer matrix results in significant improvement of the properties of polymeric materials, including mechanical properties,1-7-1 barrier properties,1-8-1 resistance to solvent swelling,[9] ablation performance,1-10-1 thermal stability,[11] fire retardancy,[11] controlled release of drugs,[12] anisotropic electrical conductivity,1-13-1 and photoactivity.[14] Layered-silicate nanocomposites have great potential for applications, ranging from automotive and aerospace to food packaging and tissue engineering. Cost and processability are as important to many applications as the property improvements.
Epoxy materials have been widely used in adhesives, coatings, electronic encapsulants, and advanced composites. Since the Pinnavaia group first extended the nano-composite concept to epoxy systems,[15] extensive research on layered-silicate epoxy nanocomposites has been carried out.[16-45] This paper is the summary of some of the past research and latest research in our group, which focus on layered-silicate epoxy nanocomposites for aerospace applications. The aerospace epoxy used in the study is made from Shell Epon 862 with Epi-Cure curing agent W. This epoxy system has a high glass transition temperature (Tg), good mechanical and physical performance characteristics, and low viscosity, and involves non-4,4′-methylenedianiline aromatic amines. The compatibility of organoclays with aerospace epoxy resin and the preparation of the nanocomposite were studied. Small-angle x-ray scattering (SAXS) and transmission electron microscopy (TEM) were used to characterize the morphology of the nanocomposites. The properties of the nanocomposite, including the dynamic and static mechanical property and survivability in aggressive environments such as oxygen plasma, were measured. The integrated study of in situ SAXS, differential scanning calorimetry (DSC), and rheology provides information about the viscosity development and processing window, as well as the morphology development and exfoliation mechanism. Morphology development behavior for the epoxy nano-composites as a function of curing agents, including Jeffamine and curing agent W, is discussed. In addition, epoxy nanocomposites as primer layer for aircraft coatings for improved anticorrosion properties are described.
LAYERED SILICATE AND LAYERED ORGANOSILICATE
There are many layered silicates, including montmoril-lonite, illite, vermiculite, hectorite, beidellite, magadiite, kenyaite, kanemite, and crystolite. Among them, mont-morillonite is the most popular choice for layered-silicate polymer nanocomposites. Natural sodium montmoril-lonite is an aluminosilicate composed of two silica tet-rahedral sheets and one alumina octahedral sheet with strong covalent bonding within the sheets and weak bonding between the layers. Sodium montmorillonite is hydrophilic in nature and is not compatible with generally hydrophobic organic polymers. However, the weak bonding between the layers makes the cations in the gallery of the layered silicate easily exchangeable. Cations are typically used for nanocomposites with alkyl ammonium. The organic ammonium pendent group on these exchanged cations renders the layered silicate hydrophobic, thus compatible with the polymer matrix. One measure of the hydrophilic or hydrophobic nature of materials is the value of surface tension (electron donor).[46] Materials that have a gLW (Liftshitz-van der Waals) value of about 40 mJ/m2 with higher than 28 mJ/m2 are hydrophobic; at smaller than 28 mJ/m2, they are hydrophobic.1-46-1 For Wyoming sodium montmorillonite (SWy-1), the gLW and values are 42.9 and 36.7 mJ/m2; thus this material is hydrophilic in nature and is incompatible with epoxy. When the native sodium cation is exchanged with an ammonium cation, the charged end of the ammonium cation maintains contact with the silicate nanolayer surface, whereas the alkyl tail is directed away from the silicate nanolayer surface. This results in a reduction of value. The actual reduction depends on the alkyl chain length. For SWy-1, the gLW and values are 41.7 and 36.2 mJ/m2 for NH4+-montmorollonite, 41.2 and 9.8 mJ/m2 for n-C6H12NH3+-montmorollonite, 39.4 and 7.7 mJ/m2 for n-C8H17NH3+-montmorollonite, and 39.6 and 6.0 mJ/m2 for n-C12H25NH3+-montmorollonite.[46] Thus it appears that the organoclay with six-carbon alkyl chain length can still be compatible with epoxy, although it is generally thought that longer alkyl chains in the pendent group are preferred for compatibility with polymers.
Based on this, an investigation of the effect of al-kyl chain length on interplanar spacings of layered-silicate epoxy nanocomposites was conducted on four synthetic organoclays, including SC6 (n-C6H13NH3+-montmorillonite), SC8 (n-C8H17NH3+-montmorillonite), SC12 (n-C12H25NH3+-montmorillonite), and SC18 (n-C18H37NH3+-montmorillonite), and on one commercial organoclay, I.30E, from Nancor. Organoclays SC6, SC8, SC12, and SC18 were prepared by the treatment of sodium montmorillonite with HCl and primary amine (n-hexylamine or n-octylamine or n-dodecylamine or n-octadecyl amine, respectively). Wide-angle x-ray diffraction (WAXD) studies show that the interplanar spacings of original sodium montmorillonite (SNA), SC6 (n-C6H13NH3+-montmorillonite), SC8 (n-C8H17NH3+-mont-morillonite), SC12 (n-C12H25NH3+-montmorillonite), and SC18 (n-C18H37NH3+-montmorillonite) are 12.1, 13.0, 13.2, 16.5, and 18.7 A, respectively. The interplanar spacings of these organoclays increase with increasing alkyl chain length of the pendent group.
AEROSPACE EPOXY NANOCOMPOSITES
There are three typical morphologies when a polymer is associated with organically modified layered silicates:[1-5] 1) traditional microcomposite—the polymer chain cannot penetrate inside the gallery of the layered silicate, resulting in a phase-separated composite; 2) intercalated nanocomposite—the polymer chains penetrate inside the gallery of the layered silicate and expand the nanolayers, resulting in a well-ordered multilayer morphology with alternating polymeric and silicate layers; and 3) exfoliated nanocomposite—the individual silicate nanolayers are completely separated and uniformly dispersed in a continuous polymer matrix. This is an ideal situation and perfect morphology. Among the most reported exfoliated nanocomposites, there exist mixed morphologies with combinations of exfoliation and intercalation. The morphology can be determined by x-ray diffraction and transmission electronic microscopy.
There are three main approaches to fabricating layered-silicate polymer nanocomposites:[3,4] 1) in situ intercala-tive polymerization—the organosilicate is swollen in the liquid monomer, the monomer penetrates inside the gallery of the layered silicate, and the polymerization takes place inside the gallery; 2) exfoliation adsorption (solvent-assisted)—the organosilicate is mixed with the solvent, which can penetrate inside the gallery of the organosilicate, enlarge the gallery of the silicate nano-layers to expansion, or delaminate the silicate nanolayers. Simultaneously, the polymer can also dissolve in this solvent. When the mixture of organosilicate and solvent is combined with the polymer, the polymer can penetrate inside the gallery of the silicate nanolayers. The solvent can then be evaporated, leaving the nanocomposite; and 3) melt intercalation—the polymer is heated into the molten state and then mixed with organosilicate. The polymer will penetrate inside the gallery of the silicate nanolayers to expand the gallery to form the nanocomposite. In situ intercalative polymerization involves chemical reactions, whereas exfoliation adsorption and melt intercalation are primarily physical. Epoxy nanocomposites are generally prepared by in situ intercalative polymerization.[15-45]
The aerospace epoxy used in the study to be referenced is made from Shell Epon 862 (diglycidylbisphenol-F) with Epi-Cure curing agent W (diethyltoluenediamine). The desired amount of organoclay was added to the Epon 862. The organoclay/Epon 862 mixture was stirred at elevated temperature, then the stoichiometric amount of curing agent W was added to the mixture. The resulting mixture was degassed, cast in the mold, and cured at 121 °C for 2 hr and at 177°C for another 2 hr, then cooled to room temperature overnight. DSC studies confirm that all of the nanocomposites were fully cured. All the organoclays, including SC6, SC8, SC12, SC18, and I.30E, can form transparent nanocomposites with the epoxy resin confirming the good compatibility of these organoclays with the Epon 862/W system. It appears that that the six-carbon alkyl chain length can render the clay nanolayers sufficiently hydrophobic to be compatible with the epoxy resin, consistent with what the of the SC6 indicates.
MORPHOLOGY CHARACTERIZATION
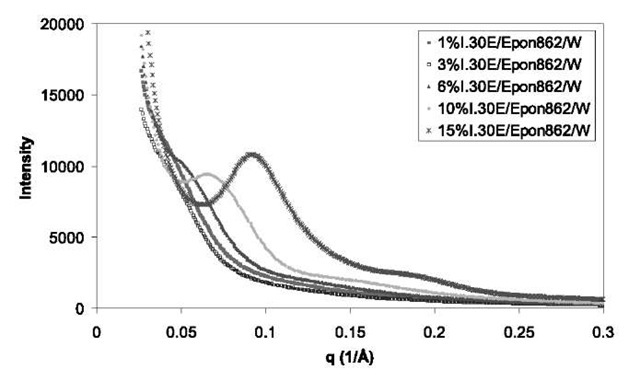
WAXD studies show no peak with 20 down to 2° for all of the aerospace epoxy nanocomposite samples even with organoclay loading as high as 15%, indicating a large interplanar spacing (>44 A). The SAXS of Epon 862/ curing agent W with different concentrations of I.30E (1%, 3%, 6%, 10%, and 15%) is shown in Fig. 1. SAXS study indicates that the interplanar spacings of all the nanocomposites with 3% and 6% different organoclay loadings are more than 100 A, and are 88 and 69 A for 10% I.30E/Epon 862/W and 15% for I.30E/Epon 862/W nanocomposites, whereas the original interplanar spacing of the organoclays is in the range of 13-23 A. The large increase in the interplanar spacing is because of the penetration of the epoxy resin inside the gallery and the expansion of the gallery of the organoclay. This also demonstrates that the dispersion of the nanolayers of the organoclay in the epoxy matrix is pretty good. Even at 10% I.30E loading, which is generally considered a very high loading in nanocomposites, the SAXS data still indicate good dispersion of clays in the matrix.
Fig. 1 SAXS of Epon 862/curing agent W with different concentrations of I.30E (1%, 3%, 6%, 10%, and 15%).
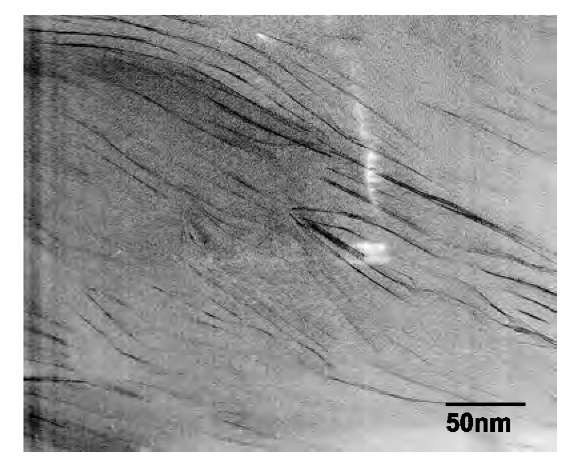
TEM provides direct imaging of the morphology of nanocomposites. The TEM image of cured 10% I.30E/ Epon 862/W nanocomposites is shown in Fig. 2. The dark lines are the cross sections of the silicate nanolayers. The original aggregates of the organoclay were disrupted, and the nanometer-thick individual sheets were well dispersed in the epoxy resin. Although some nanolayers are disordered, others preserve the original ordered structure separated by approximately 10 nm, consistent with the SAXS results. The image also indicates that there still exist some ordered structures with disordered structure and some pure polymer domains. The TEM image of the cured 3% SC6/Epon 862/W nanocomposite is shown in Fig. 3. The interplanar spacing between the nanolayers of the ordered gallery is ~ 15 nm, consistent with the SAXS results (a weak 001 reflection at 140 A). The dispersions of the layers are very good. However, it is still not strictly an exfoliated nanostructure; rather, it is partially exfoliated with some intercalated structures of very large interplanar spacings 15 nm).
Fig. 2 TEM image of cured 10% I.30E/Epon 862/W nanocomposite.
Fig. 3 TEM images of cured 3% SC6/Epon 862/W nanocom-posite.
PROPERTIES OF AEROSPACE EPOXY NANOCOMPOSITES
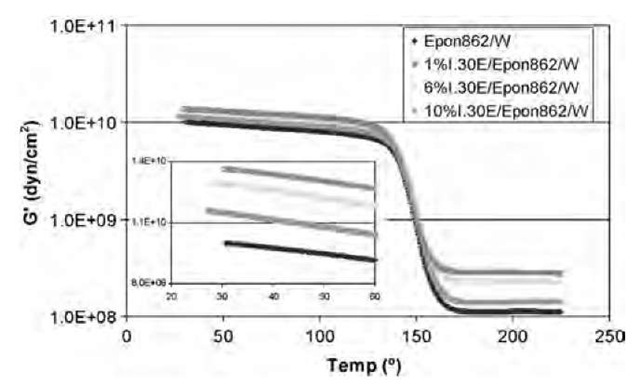
The storage modulus and tan d curves obtained through dynamic mechanical analysis (DMA) of pure Epon 862/W and 1% I.30E/Epon 862/W, 6% I.30E/Epon 862/W, and 10% I.30E/Epon 862/W nanocomposites are shown in Figs. 4 and 5. The DMA data indicate that the increase in the storage modulus of the nanocomposites with different organoclays (1%, 3%, 6%, 10%, and 15% I.30E; 3% SC6; 6% SC8; 6% SC12; and 6% SC18) ranges from 15% to 35% in the glass state and from 15% to 300% in the rubber state. The increase of the storage modulus is attributed to the high aspect ratio and relatively high stiffness of the silicate nanolayers in the epoxy matrix. Based on the tan d curve, the glass transition temperatures of the nanocom-posites are from 152°C to 155°C, compared with 154°C of the pure Epon 862/W, except for the 143°C for the 15% I.30E/Epon 862/W nanocomposite. The decrease for the 15% I.30E/Epon 862/W nanocomposite perhaps is caused by the less homogeneous dispersion of the organoclay because of the high concentration of I.30E in the nanocomposite and plasticization effect from the organoclay.
Fig. 4 Storage modulus vs. temperature of cured pure Epon 862/W and 1% I.30E/Epon 862/W, 6% I.30E/Epon 862/W, and 10% I.30E/Epon 862/W nanocomposites.
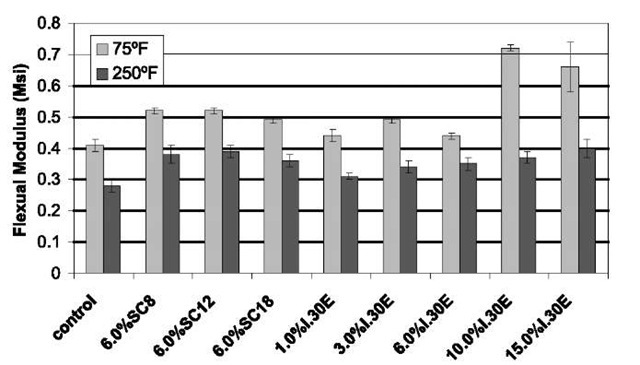
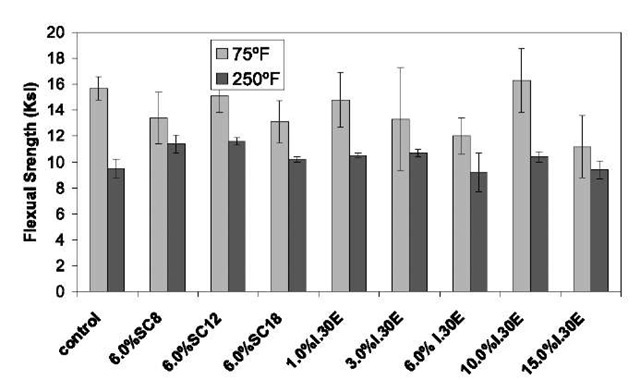
Flexural data from three-point flex testing performed at room temperature and at high temperature (250°F) are shown in Figs. 6 and 7. The flexural modulus of the epoxy is significantly increased because of the organoclays by as much as 85%, with 10% I.30E loading. The improvement in modulus is perhaps because of the good dispersion of the stiffer clay nanolayers in the epoxy matrix, which may transfer and carry more load. However, the strength of the nanocomposites is decreased compared with that of the pristine epoxy resin. The results are similar to the those for the epoxy system reported by Zerda and Lesser[26] and Yasmin et al.[44] Strength is more sensitive to the interface and to defects than modulus. The small reduction of strength may be partially attributed to the weak interface between the clay nanolayer and the epoxy because there is no covalent bonding in the interface between the nanoclay and the epoxy matrix. One of the key factors to improving the strength of the nanocomposite is the enhancement of the interfacial interaction between the polymer matrix and the layered silicates. Another possible reason for the reduction is the possible existence of nanovoids inside the nanocomposite system.[44] The data also show that the reduction of flex strength of the nanocomposites at elevated temperature is much smaller than that seen by the pristine polymer. Interestingly, the strength of some of the nanocomposites is higher than that of pure resin at 250°F. The cause for this significant phenomenon is under investigation.
Fig. 5 Tan d curve of cured pure Epon 862/W and 1% I.30E/ Epon 862/W, 6% I.30E/Epon 862/W, and 10% I.30E/Epon 862/W nanocomposites.
Fig. 6 Flexural modulus of the pure Epon 862/W and nanocomposites at room temperature and 250°F.
The survivability of the nanocomposites in extreme environments may be improved and was assessed here via exposure to oxygen plasma.[47] Oxygen plasma contains many species that are extremely strong oxidants and to which polymeric materials are vulnerable. The surface erosion thicknesses after a 5-hr exposure to oxygen plasma are 34 and 22 mm for the pure Epon 862/W and 6% for the I.30E/Epon 862/W nanocomposites, respectively, when they were exposed to oxygen plasma. Under this environment, the surface erosion rate of the nanocompo-site is retarded compared with that of pure epoxy resin. A possible mechanism for the improved survivability is the ability of the nanocomposite to form intact inorganic layers when exposed to oxygen plasma.[47] The preferential oxidation of polymers and the corresponding enrichment of the nanoscale-layered silicates on the surface result in the formation of a ceramic-like inorganic layer, which retards the penetration of the oxygen plasma, thus slowing further degradation. Thus the incorporating organoclays in polymer matrices could increase the survivability of resulting composites in aggressive aerospace environments.
Fig. 7 Flexural strength of the pure Epon 862/W and nanocomposites at room temperature and 250°F.