Polycythemia Vera
Early studies in untreated PV patients found a high incidence of thrombotic events and a life expectancy of about 18 months after diagnosis (Chievitz and Thiede 1962). Cytoreductive treatments of blood hyperviscosity by phlebotomy or chemotherapy have dramatically reduced the number of thrombotic events, even though hematological transformations towards post-polycythemic myelofibrosis and acute leukemia still represent a major cause of death (Marchioli et al. 2005). Since there is a concern that myelosuppressive drugs given to control the proliferative phase of the disease might be implicated in the long-term complications, current treatment recommendations should be adapted on the expected risk for thrombosis of the patient.
Incidence and Type of Thrombosis and Hemorrhage
At initial presentation, the reported incidence of thrombosis and bleeding in PV patients varied from 12-39% to 1.7-20%, respectively.Factors that could have accounted for the relatively wide range of values include patient selection, definition of events, and accuracy in data reporting. The largest epidemiologic study (European Collaboration on Low-dose Aspirin, ECLAP) included 1,638 patients followed for a median of 2.8 years (Marchioli et al. 2005). A total of 164 deaths (10%) were recorded for an overall mortality rate of 3.7 per 100 persons per year. As compared with the general Italian population standardized for age and sex, the excess of mortality of PV patients was 2.1 times. Cardiovascular mortality accounted for 41% of all deaths (1.5 deaths per 100 persons per year), mainly due to large vessel arterial events, such as coronary heart disease and non-hemorrhagic stroke. The cumulative rate of non-fatal thrombosis was 3.8 events per 100 persons per year, without difference between arterial and venous thrombosis. Major and fatal bleeding were rare, accounting for only 0.8 and 0.15 events per 100 persons per year. Thus, thrombosis represents a major cause of morbidity and mortality in PV.
Particularly serious thrombotic events typically associated with PV are abdominal vein thrombosis (AVT), including Budd-Chiari syndrome and obstruction of the portal, mesenteric, and splenic system. Overall, PV and other myeloproliferative neoplasms (MPN) were reported to be the major cause of AVT, accounting for 25-65% of cases.Of importance, the diagnosis of MPN may be difficult in these disorders because blood cell counts and serum erythropoietin levels can be still within the normal limits or only slightly modified, and the presence of a splenomegaly is of little diagnostic value. Specialized tests, including bone marrow biopsy and endogenous erythroid colony formation, have been advocated (Chait et al. 2005), but a step forward in the diagnosis of an occult MPN in these patients has been done with the discovery of JAK2 V617F. Testing for the mutation was found positive in 40-58% of patients with Budd-Chiari syndrome and 36% of those with portal vein thrombosis (Patel et al. 2006; Primignani et al. 2006) and is now recommended in all patients presenting with AVT (Chung et al. 2006).
Risk Stratification According to Thrombotic Risk
Age and Previous Thrombosis
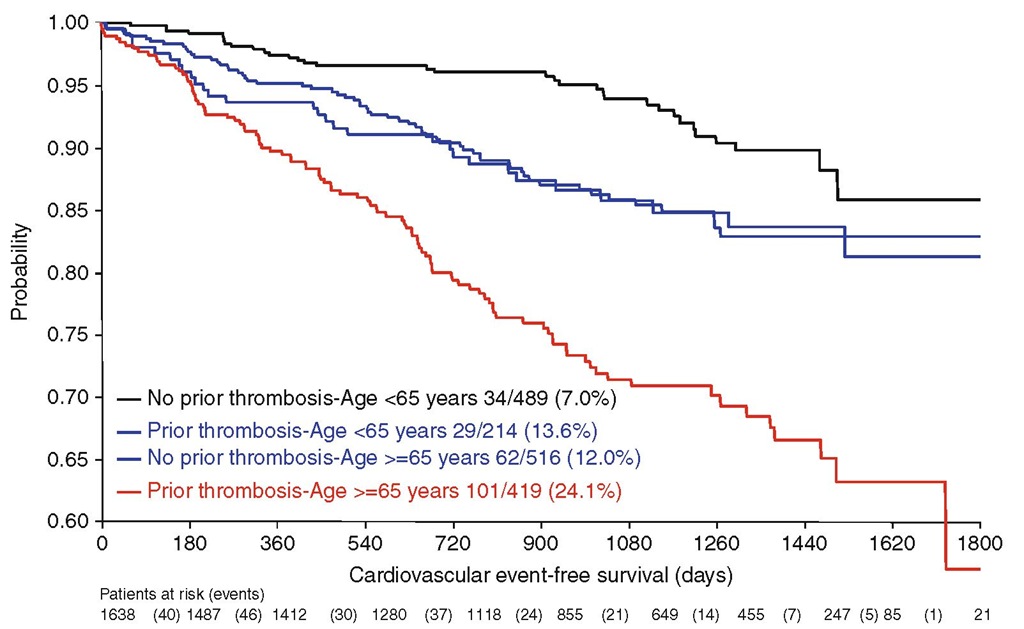
Increasing age and a history of vascular events have consistently proven to be independent predictors of thrombosis in patients with PV (Berk et al. 1986; Marchioli et al. 2005). The first to report these risk factors were the Polycythemia Vera Study Group (PVSG) investigators in a multivariate analysis of the entire cohort of 431 patients enrolled in the seminal 01 clinical trial. Phlebotomy treatment, rate of phlebotomy, history of prior thrombosis and advanced age contributed significantly to the overall risk of thrombosis, whereas this was not found for other parameters such as sex and pre-treatment hematological indices (i.e. hematocrit, white cell, and platelet count) (Berk et al. 1986). In the ECLAP study, the incidence of cardiovascular complications was higher in patients aged more than 65 years (5.0% patient-year, hazard ratio 2.0, 95% confidence interval [CI] 1.22-3.29, P < 0.006) or with a history of thrombosis (4.93% patient-year, hazard ratio 1.96, 95% CI 1.29-2.97, P=0.0017) than in younger subjects with no history of thrombosis (2.5% patient-year, reference category) (Fig. 7.1) (Marchioli et al. 2005) . At variance of the PVSG findings, performance of phlebotomy was not associated with an increased thrombotic risk (relative risk 0.89, 95% CI 0.67-1.18).
The Role of Hematocrit
The principal hemorrheologic abnormality in PV is an elevated whole blood viscosity. The blood viscosity in PV is higher than that of normal controls at all shear rates (Thomas et al. 1977). In a retrospective analysis of the records of 69 PV patients with histories of vascular thrombosis, Pearson and Wetherley-Mein (1978) demonstrated a strong correlation in univariate analysis between hematocrit level and the development of thrombotic episodes, including many cerebrovascular occlusions. Thomas and coworkers (1977) showed that cerebral blood flow is reduced in patients with PV in whom the hematocrit is 53-62%. These abnormalities were observed even in patients with hematocrits at the lower levels of normal, that is, 46-52%. Reductions in cerebral blood flow were correctable with phlebotomy. Reduction of the hematocrit by relatively small amounts frequently led to substantial improvements in whole blood viscosity and cerebral blood flow. Some PV patients apparently still maintain a higher than normal whole blood viscosity despite the normalization of the hematocrit, suggesting that an increase in hematocrit may not be the only factor responsible for increased blood viscosity.
Fig. 7.1 Rate of vascular complications during follow-up in 1,638 patients with PV enrolled in the ECLAP study according to prior thrombosis and age
Almost all patients with PV are iron deficient. Decreased red cell deformability has been said to accompany iron deficiency, leading to increased blood viscosity and a decreased ability of red cells to pass through small-bore polycarbonate filters (Hutton 1979). Such abnormalities have been shown to be due to increased membrane stiffness rather than to reduced surface-volume ratio (Yip et al. 1983). Studies of patients with hemoglobinopathies due to abnormal oxygen binding who have secondary erythrocytosis provide further support to the belief that hemat-ocrit elevations are not the sole cause of the thrombotic tendency in PV. A survey of 200 patients with these types of hemoglobinopathies has not demonstrated a higher incidence of myo-cardial ischemia or any other form of thrombosis, even though the red cell mass is frequently as elevated as that in patients with PV (Bunn and Forget 1986). Furthermore, Shibata and colleagues studied the risk of thrombosis in a trans-genic mouse model with extreme erythrocytosis due to over-expression of EPO and did not observe an increased incidence of thrombosis (Shibata et al. 2003).
The issue of hematocrit and thrombosis in PV has been recently updated. In the ECLAP study, despite the recommendation of maintaining the hematocrit values at less than 0.45, only 48% of patients had values below this threshold, whilst 39% and 13% of patients remained between 0.45 and 0.50 and greater than 0.50, respectively (Marchioli et al. 2005) . Multivariate models considering all the confounders failed to show any correlation between these hematocrit values and thrombosis (Di Nisio et al. 2006) . A total of 164 deaths (10%), 145 (8.85%) major thrombosis and 226 (13.8%) total thrombosis were encountered during 4,393 person-years of follow-up (median 2.8 years). An association between relevant outcome events (thrombotic events, mortality, and hematological progression) and hematocrit in the evaluable range of 40-55% was found neither in the multivariate analysis at baseline nor in the time-dependent multivariate model (Di Nisio et al. 2006). For the time being, the recommended hematocrit target is below 45%,but the uncertainty described above prompted Italian investigators to launch a prospective, randomized clinical study (CYTO-PV) addressing the issue of the optimal target of cytoreduction in PV (EudraCT 2007-006694-91).
Platelet Number and Function
No study to date has demonstrated a significant correlation between platelet number or function and thrombosis. In the PVSG 01 clinical trial, platelet counts did not predict thrombosis, either measured at baseline or at the nearest times before the thrombotic event (Berk et al. 1986). Accordingly, an ad hoc analysis of the patients enrolled in the ECLAP study failed to show any association between platelet count and throm-botic events (Di Nisio et al. 2006). Neither the currently proposed therapeutic target of 400 χ 109/L nor any other of the platelet count thresholds evaluated in this analysis predicted a higher risk of thrombosis. The concordant findings of these two major prospective studies in PV suggest that current treatment does not primarily aim at lowering the platelet count.
Qualitative platelet abnormalities occur frequently in PV patients. Increased plasma and urinary thromboxane production has been linked to increased platelet activation (Landolfi et al. 1992). A low-dose aspirin regimen selective for inhibition of platelet cyclooxygenase has been found to suppress increased throm-boxane production in vivo and to clinically benefit patients with PV (GISP 1997; Landolfi et al. 2004). In addition, selected patients with PV have been afforded prompt resolution of vascular complications such as erythromelalgia or transient ischemic attacks following institution of platelet antiaggregating agents (Michiels et al. 1985) . It is important to emphasize that erythromelalgia does not resolve in PV patients with phlebotomy alone or with anticoagulation but requires the use of platelet antiaggregating agents, namely aspirin. Although a variety of clinical assessments of platelet function have been used to identify patients who are potentially at a high risk of developing a life-threatening hemorrhagic or thrombotic event, the results of these studies to date have been very disappointing. It appears that the etiology of thrombosis and hemorrhage in PV is multifac-torial and that the available tools are inadequate to identify those patients at highest risk.
Leukocytes
Leukocytosis was found to be an independent risk factor for thrombosis.PV patients with WBC count >15,000 χ 109/L, compared to those with WBC count <10,000 χ 109/L, had a significant 70% increase of the rate of vascular complications, mainly represented by myo-cardial infarction (Landolfi et al. 2007). Similar findings have been reported in patients with ET (see below). Activated leukocytes may release proteases and oxygen radicals which alter endothelial cells and platelets so as to favor the development of a prothrombotic state. A series of markers of leukocyte activation, including expression of membrane CD11B and leukocyte alkaline phosphatase antigen, cellular elastase content, plasma elastase levels, and myeloperoxidase levels are elevated in patients with PV (Falanga et al. 2005). An association between these functional abnormalities and the presence of JAK2 mutation has been reported (Arellano-Rodrigo et al. 2006). Overall, these results would indicate that an increased release of neutrophil proteases may provide a mechanism by which the homeostatic pathway is activated in PV, which ultimately would contribute to the establishment of a pre-thrombotic state (Marchetti et al. 2008).
Other Risk Factors
Conventional risk factors for atherosclerosis, including hypertension, hyperlipidaemia, diabetes, and smoking, have been assessed in PV with variable results, possibly reflecting the size of the studies and number of patients with the risk markers of interest included. In the absence of consistent data, it seems reasonable to assume that common cardiovascular risk factors are associated in PV with the same relative risk as those estimated in the general population. According to this assumption, recent guidelines recommend that all patients should be managed aggressively for their risk condition and should be requested to stop smoking.
Several studies have explored the contribution of inherited and acquired thrombophilic states to the occurrence of thrombotic events in PV with some conflicting results. To date, there is no convincing evidence that identification of a thrombo-philic abnormality adds to the management of patients, so that routine thrombophilia screening is not currently recommended.
The influence of the JAK2 V617F mutational load on the thrombotic risk has been evaluated in 173 patients with PV (Vannucchi et al. 2007). The mean mutant allele burden was 52%, with 32 patients (18%) having greater than 75% mutant allele. The burden of JAK2 V617F allele correlated with measurements of stimulated erythro-poiesis (higher hematocrit, lower mean cell volume, serum ferritin, and erythropoietin levels) and myelopoiesis (higher white cell count, neu-trophil count, and serum lactate dehydrogenase) and with markers of neutrophil activation (elevated leukocyte alkaline phosphatase and PRV-1 expression). As compared to those with less than 25% mutant allele, patients harboring greater than 75% JAK2 V617F allele were at higher relative risk (RR) of developing major cardiovascular events during follow-up (RR 7.1; P = 0.003).
Essential Thrombocythemia
A risk stratification based on thrombohemorrhagic risk is needed also for patients with ET, as for those with PV, with the purpose to focus the indication for myelosuppressive therapy on high-risk patients only and to avoid the risk of over-treatment in individuals with a low probability to develop major bleeding or thrombotic complications.
Incidence and Type of Thrombosis and Hemorrhage
Thrombosis and hemorrhage are the most frequent clinical complications observed in ET patients. In uncontrolled studies, reported cumulative rates for thrombosis and hemorrhage during follow-up ranged from 7% to 17% and 8% to 14%, respectively.In one study that also evaluated a control population (Cortelazzo et al. 1990), the incidence of thrombotic episodes was 6.6% per patient-year in ET vs. 1.2% in control subjects, and the rate of major hemorrhagic complications was 0.33% per patient-year in ET vs. 0% in controls.
The most frequent types of major thrombosis include stroke, transient ischemic attack, myocar-dial infarction, peripheral arterial thrombosis, and deep venous thrombosis often occurring in unusual sites, such as hepatic (Budd-Chiari syndrome), portal, and mesenteric veins. In addition to large vessel occlusions, ET patients may suffer from microcirculatory symptoms, including vascular headaches, dizziness, visual disturbances, distal paraesthesia, and acrocyanosis cases.The most characteristic of these disturbances is erythromelalgia, consisting of congestion, redness, and burning pain to ischemia and gangrene of distal portions of toes and fingers (Michiels et al. 1985) . The most frequent bleeding events are hemorrhages from the gastrointestinal tract followed by hematuria and other muco-cutaneous hemorrhages. Hemarthrosis and large muscle hematomas are uncommon.
Risk Stratification According to Thrombotic Risk
Age and Previous Thrombosis
Age over 60 and a previous thrombotic event were identified as major risk factors for thrombosis in most studies (summarized in Table 7.1). The age-related differences in the frequency of these events have been attributed to the coexistence of vascular disease in older patients (Watson and Key 1993). However, younger patients are not free of vascular thrombosis, sometimes in unusual sites, such as the portal or sagittal veins (Mitus et al. 1990). Overall, the incidence of thrombotic and hemor-rhagic complications in asymptomatic patients with essential thrombocythemia less than 60 years of age who had a platelet count of less than 1,500 χ 109/L has been shown to be comparable with a normal control population (Ruggeri et al. 1998). These authors have emphasized the importance of the concurrent absence of both of the formerly mentioned clinical characteristics to define a low-risk profile in young patients with essential thrombocythemia.
Platelet Count
Several large cohort studies have failed to define a relationship between the frequency of thrombotic complications and platelet number (Table 7.1) . Thus, an elevated platelet count, per se, should not be considered an indication for a myelosuppressive therapy aimed at preventing thrombotic complications. Supporting this view, in a retrospective study of 99 consecutive young patients (aged <60 years) who presented with extreme thrombocytosis (platelet count >1,000 χ 109/L) and without a previous history of thrombohemorrhagic complications, the incidences of major thrombosis and hemorrhage during the follow-up were similar between those who were treated with prophylactic cytoreductive therapy and those who did not receive such therapy.
The relationship between frequency of bleeding episodes and high platelet counts is much more consistent. Several studies have shown that the degree and duration of bleeding in this patient population correlate with the platelet count (Table 7.1). Bleeding events appear to occur exclusively when the platelet counts are excessively high and stop when the platelet count falls to normal. The clinical spectrum of bleeding in essential thrombocythemia patients closely resembles that observed in von Willebrand disease (Budde et al. 1993; van Genderen et al. 1996) . Several groups have now shown that high platelet counts (>1,000 χ 109/L) are associated with an acquired von Willebrand syndrome and that reduction of platelet numbers is associated with correction of the von Willebrand abnormalities and cessation of bleeding episodes (Budde et al. 1993; van Genderen et al. 1996). An increase in the number of circulating platelets appears to favor the adsorption of larger von Willebrand multimers onto platelet membranes, resulting in their removal from the circulation and their subsequent degradation. The laboratory features of acquired von Willebrand syndrome in essential thrombo-cythemia is characteristic of a type II deficiency with a prolonged bleeding time, decreased ristocetin cofactor activity, and a decrease or absence of large von Willebrand factor multimers.
Platelet Function
Platelets in patients with essential thrombo-cythemia have been known for a considerable time to be qualitatively abnormal (Landolfi et al. 2006; Finazzi et al. 2009) . Although both increased and decreased platelet reactivities have been described, these findings have not been definitively associated with thrombohemorrhagic complications with two noteworthy exceptions; erythromelalgia, where the prompt relief of symptoms by cyclooxygenase inhibitors provides direct evidence that prostaglandins play a role in the development of vascular occlusion (Michiels et al. 1985/ . and acquired von Willebrand syndrome, which is a major cause of bleeding in patients with essential thrombocythemia (Budde et al. 1993; van Genderen et al. 1996).
Prolongation of the bleeding time has been reported in 7-19% of newly diagnosed patients with essential thrombocythemia (Finazzi et al. 2009) . A close correlation between prolongation of the bleeding time and the occurrence of hemorrhage in essential thrombocythemia patients does not always exist. This is in contrast to correction of the bleeding time in those patients with essential thrombocythemia, extreme thrombocy-tosis, and acquired von Willebrand syndrome following reduction of platelet numbers to the normal range. It is important to emphasize that aspirin prolongs the bleeding time of patients with essential thrombocythemia to a greater degree than that of normal controls (Budde et al. 1993; van Genderen et al. 1996).
Abnormal platelet aggregation has been reported in 35-100% of patients with essential thrombocythemia. Abnormal aggregation studies are not related to prolongation of the bleeding time or to the incidence of episodes of hemorrhage or thrombosis. In essential thrombo-cythemia, platelet aggregation is classically defective in response to epinephrine, adenosine diphosphate (ADP), and collagen but is usually normal with arachidonic acid and ristocetin. Characteristically, in essential thrombocythemia, the first wave of aggregation is diminished, and the second wave of aggregation is absent in response to epinephrine. An acquired form of platelet storage pool disease occurs frequently in essential thrombocythemia. Platelet alpha-granule content and release are abnormal, resulting in elevated plasma levels of platelet factor-4 and beta-thromboglobulin. Because the content of alpha-granule constituents has been reported to be normal in megakaryocytes isolated from essential thrombocythemia, the synthesis of these molecules is not believed to be abnormal, but rather, the release of alpha-granule constituents is believed to be a consequence of platelet activation. The finding of an acquired storage pool defect again does not correlate with platelet numbers or with the occurrence of clinical symptoms (Finazzi et al. 2009) .
Table 7.1 Cohort studies of risk factors for thrombosis and bleeding in essential thrombocythemia including at least 100 patients
|
Study (ref) |
Patients, no. |
Risk factors for thrombosis (RR or P) |
||||
|
Age > 60 |
Previous thrombosis |
Platelet count |
Leukocytosis |
Cardiovascular risk factors3 |
||
|
Cortelazzo et al. (1990) |
100 |
10.3 (2.05-51.5) |
13 (4.1—41.5) |
NS |
- |
NS |
|
Besseset al. (1999) |
148 |
3.3 (1.5-7.4) |
3.0(1.5-6.0) |
NS |
- |
4.7 (1.8-11.8) |
|
Colombi et al. (1991) |
103 |
NS |
Ρ <0.001 |
NS |
- |
- |
|
Jantunen et al. (2001) |
132 |
NS |
- |
NS |
- |
P=0.01 |
|
Bazzan et al. (1999) |
187 |
NS (age >55) |
- |
NS |
- |
NS |
|
Wolanskyi et al. (2006) |
322 |
1.51 (1.05-2.18) |
2.3 (1.25-4.24) (arterial only) |
- |
1.74 (1.15-2.66) (WBC > 15 χ 109/L) |
NS |
|
Carobbio et al. (2007) |
439 |
2.3 (1.3-3.9) (age >60 and previous thrombosis evaluated together) |
NS |
2.3 (1.4-3.9) (WBC > 8.7 χ 109/L) |
- |
|
|
Passamonti et al. (2008) |
605 |
Ρ <0.001 P=0.03 |
NS |
NS |
- |
|
|
Palandri et al. (2011) |
532 |
2.45 (1.35-4.43) |
NS |
1.76 (1.95-2.97) (WBC > 11 χ 109/L) |
— |
|
|
Study (ref) |
Patients, no. |
Risk factor for bleeding |
||||
|
Platelet count |
||||||
|
van Genderen and Michiels (1994) |
200 (review of published cases) |
P<0.001 (platelets >1,000 χ 109/L) |
||||
|
Fenaux et al. (1990) |
147 |
"Higher risk" (platelets>2,000χ 109/L) |
||||
|
Wolanskyi et al. (2006) |
322 |
NS |
||||
|
Alvarez-Larran et al. (2010) |
300 |
P<0.01 (platelets > 800 xl09/L) |
||||
NS not significant
aAt least one of the following: smoking, hypertension, hypercholesterolemia, diabetes
The survival of platelets in patients with eryth-romelalgia and thrombosis has been shown to be reduced to 4.2 ± 0.2 days compared with normal platelet survivals in asymptomatic essential thrombocythemia patients (6.6 ± 0.3 days) and patients with reactive thrombocytosis (8.0 ± 0.4 days) (van Genderen 1995). Thrombosis in this setting is associated with an increased platelet turnover, which can be quantitated cytometri-cally by measuring the number of platelets most recently released into the circulation (reticulated platelets). Treatment of erythromelalgia with aspirin increased mean platelet survival from 4.0 ± 0.3 to 6.9 ± 0.4 days and was associated with a significant elevation of platelet numbers (van Genderen 1995). These findings suggest that erythromelalgia results from platelet-mediated thrombosis of the arterial microvasculature of the extremities. Complete correction of this ischemic circulatory defect is associated with the use of platelet cyclooxygenase inhibitors, such as aspirin (van Genderen and Michiels 1994) . Agents that do not inhibit platelet cyclooxygenase, such as coumadin, sodium salicylate, dipyridamole, sulfinpyrazone, and ticlopidine, are not active in the treatment of this disorder (van Genderen and Michiels 1994).
One intriguing explanation for the increased risk of thrombosis in patients with essential thrombocythemia has been proposed by Lee and Baglin (1995). These investigators demonstrated that the total amount of thrombin generated on the platelet surfaces of patients with essential thrombocythemia was markedly greater than that generated on the platelet surfaces of normal controls or patients with reactive thrombocytosis. The molecular basis of this abnormality has not been defined, but it remains possible that an abnormal membrane structure of essential throm-bocythemia platelets may account for the enhanced thrombin potential that may lead to a relatively high thrombotic risk. On this ground, Trappenburg and coworkers /2009) have more recently documented increased numbers of platelet microparticles, as well as increased platelet-neutrophil and platelet-monocyte conjugates, in patients with essential thrombocythemia. Platelet microparticles support thrombin generation and leukocyte activation. Increased numbers of platelet microparticles have been associated with the development of vascular thrombosis.