JAK2V617F Mutation
The demonstration of the acquired gain-of-func-tion V617F mutation in the tyrosine kinase JAK2 gene (Baxter et al. 2005; Kralovics et al. 2005; Levine et al. 2005; Zhao et al. 2005) has greatly influenced the diagnostic and therapeutic approach in MPN patients. Several studies have implicated JAK2V617F mutation in the increased thrombotic tendency observed in ET and PV patients. A recent metanalysis that included 21 studies involving patients with ET and 6 studies with PMF showed that in ET patients, the presence of JAK2V617F mutation was associated with a significant twofold increased risk of thrombosis (OR 1.92, 95% CI 1.45-2.53), both of venous (OR 2.49, 95% CI 1.71-3.61) and arterial (OR 1.77, 95% CI 1.292.43) vessels, while its role in PMF patients is uncertain (Lussana et al. 2009).
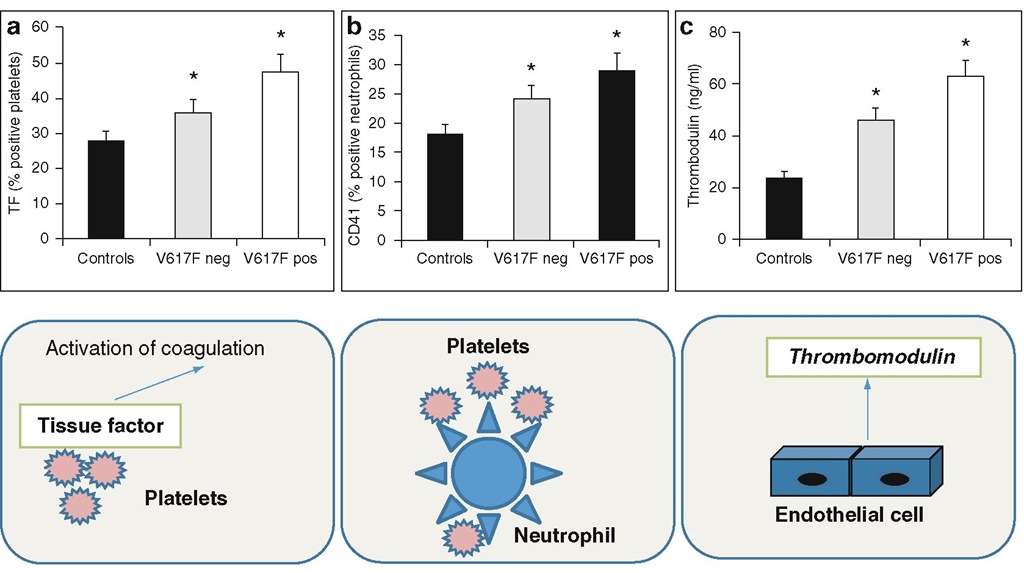
Fig. 6.3 ET patients with the JAK2V617F mutation and hemostatic system activation. ET patients carrying the JAK2V617F mutation present with significant higher expression of TF on platelet surface (panel A), circulating neutrophil/platelet aggregates (panel B), and plasma levels of the endothelial cell activation marker thrombomod-ulin (panel C). * p < 0.05 versus controls.
So far, only few studies have been published addressing whether the JAK2V617F mutation may specifically affect the hemostatic system (Alvarez-Larran et al. 2008; Arellano-Rodrigo et al. 2006; Falanga et al. 2007; Robertson et al. 2007). Altogether, these studies indicate that both cellular (i.e., platelets and leukocytes) and plasma compartments of hemostasis were more activated in those patients positive for the JAK2V617F mutation. Regarding cellular components, P-selectin was found increased on platelets from JAK2V617F-positive ET patients (Arellano-Rodrigo et al. 2006), and CD14 and LAP were demonstrated to be more highly expressed on neutrophils from ET JAK2V617F mutation carriers [54], while a significantly higher expression of CD 11b was observed on neutrophil and monocyte from JAK2V617F-positive PMF patients (Alvarez-Larran et al. 2008). In addition, our group could also demonstrate an elevated expression of TF in platelets from JAK2V617F-positive ET compared to negative patients and increased levels of plate-let/neutrophil aggregates (Falanga et al. 2007). The evidence that both platelets and neutrophils from JAK2V617F mutation carriers expressed increased activation features was in good agreement with the findings of increased mixed cell aggregate formation in ET patients carrying the JAK2V617F mutation (Fig. 6.3).
Among hypercoagulation parameters, plasma levels of soluble thrombomodulin were found to be elevated in JAK2V617F-positive ET patients (Falanga et al. 2007) , while sP-selectin levels were significantly elevated in JAK2V617F-positive ET, PV, and PMF patients compared to negative subjects (Robertson et al. 2007) . In a study by Marchetti et al. (2008) , an involvement of JAK2 mutation was observed in the presence of an acquired activated protein C resistance phenotype.
There are few studies that have evaluated the molecular mechanisms by which JAK2V617F mutation can affect the prothrombotic phenotype of a cell. It has been reported that JAK2-activating mutation can cause increased red cell adhesiveness through modification of surface adhesion molecules, facilitating thrombosis (Buss et al. 1985). In addition, JAK2 mutation may render platelet hyperresponsiveness through altered expression of cMPL signal transduction for TPO-induced platelet priming (Kubota et al. 2004). Activation of JAK2 is also involved in TF expression by neutro-phil and monocyte through the MAP and PI3 kinase pathway (Rafail et al. 2008).
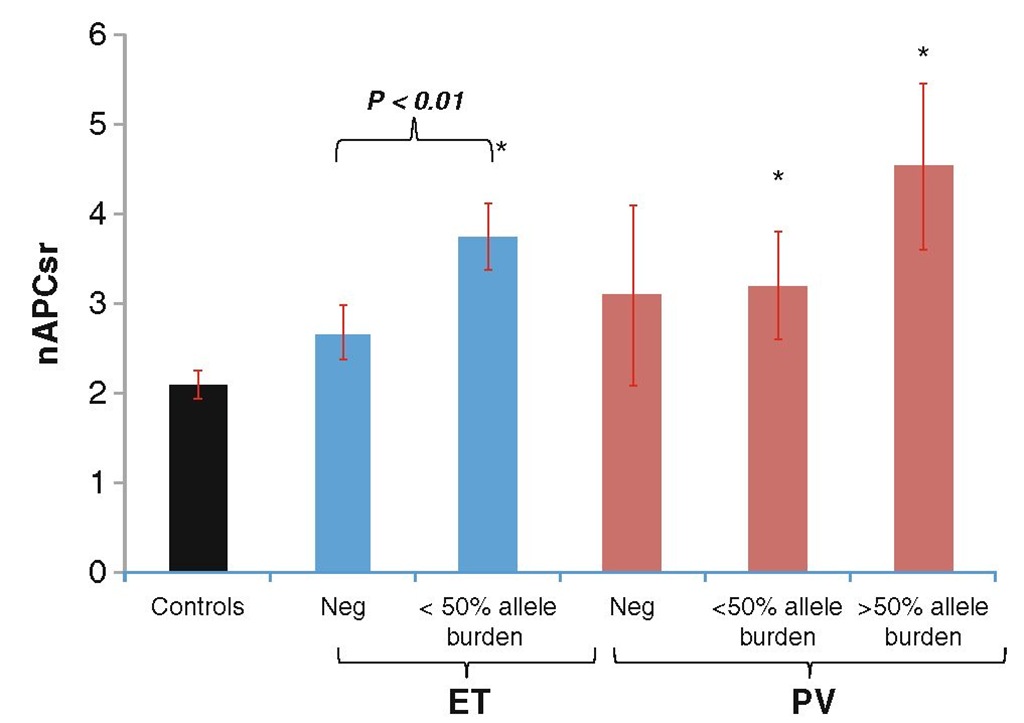
Fig. 6.4 APC resistance in ET and PV patients. APC resistance was assessed in plasma of patients with ET and PV by the thrombin generation assay in the absence and presence of APC. Data are expressed as normalized APC sensitivity ratio (nAPCsr). ET and PV carriers of the JAK2V617F mutation are more APC resistant than non-carriers, especially if homozygous (i.e., JAK2V617F allele burden >50%). * p < 0.05 versus controls.
Acquired Activated Protein C (APC) Resistance
Inherited and acquired APC resistance is associated with an increased risk of thrombosis in many conditions, including pregnancy, oral contraceptive use, hormone replacement therapy, and cancer. Decreased levels of protein C and protein S can be responsible for this phenotype, and studies reported a reduction in the concentration of natural anticoagulants in this group of patients (Bucalossi et al. 1996). By using the thrombin generation assay, our group could demonstrate the occurrence of an acquired APC resistance phenotype in ET and PV patients (Marchetti et al. 2008). The results analyzed according to the presence (i.e., positivity or negativity) and the status (i.e., heterozygosity or homozygosity) of the JAK2V617F mutation indicated that JAK2V617F mutation carriers are more APC resistant than noncarriers, especially if homozygous (i.e., JAK2V617F allele burden >50%) (Fig. 6.4). This suggests a progression in the APC-resistant phenotype determined by the JAK2V617F status and, together with previous observations, supports the hypothesis of a more hypercoagulable condition in JAK2V617F carriers. Prothrombin, factor V, free PS, and tissue factor pathway inhibitor (TFPI) levels were significantly reduced in patients, and mainly in JAK2V617F carriers. Multiple regression analysis indicated the low free PS level as a major determinant of the increased nAPCsr. Neutrophil elastase was significantly increased in all of the patients compared to controls and was significantly and inversely related to free PS plasma levels. Therefore, the increased blood cell counts, together with blood cell activation, can contribute to the chronic activation of blood coagulation and hence to the consumption of coagulation factors. A high prevalence of acquired APC resistance, in this case determined as the classical prolongation of activated partial thrombo-plastin time (aPTT) after addition of APC, was also reported by Arellano-Rodrigo et al. in a group of patients with ET (Arellano-Rodrigo et al. 2009). Again, decreased levels of free PS were detected and its inverse relationship with JAK2V617F allele percentage. In addition, acquired APC resistance was found to be more frequent in patients with previous history of thrombosis.
Plasma Microparticles
Microparticles (MPs) are membrane fragments ranging in size from 0.1 to 1 mm, released by most cell types, including blood and vascular cells, upon activation. Since microparticles are known to be elevated in thromboembolic diseases and malignancy (Zwicker et al. 2009), we hypothesized a role for microparticles in the pathogenesis of thrombosis in ET (Trappenburg et al. 2009). The levels and the cellular origin of microparticles were determined by flow cyto-metric analysis, while the microparticle-associ-ated procoagulant activity was measured using a thrombin generation assay. We found a significantly higher number of circulating microparti-cles positive for platelet and endothelial markers and for TF in ET compared to controls. In addition, microparticle-rich plasma from patients with ET has higher thrombin generation potential, which correlated significantly with the total number of microparticles. A subsequent study by Duchemin et al. I2010) showed the occurrence of an acquired "thrombomodulin resistance" phenotype in PV and ET patients, partly determined by circulating microparticles.
Conclusions
Thrombotic complications are common in patients with ET and PV and significantly impact on morbidity and mortality in these diseases. In addition, these patients commonly present with abnormalities in laboratory coagulation tests, consistent with a hypercoagulable state.
The pathogenesis of the activation of blood coagulation in these conditions is complex and includes both abnormalities of the erythrocytes, platelets, and leukocytes, arising from the clonal proliferation of hematopoietic progenitor cells.
These abnormalities involve not only quantitative changes in the number of these cells, but also involve qualitative changes in the molecular characteristics of these cells. In patients with ET and PV, measurements of neutrophil phenotypic changes and plasma parameters of neutrophil degranulation clearly show a functional activation status of these cells. These properties, together with an increased production of procoagulant microparticles and the occurrence of an APC resistance phenotype, may contribute to the clotting activation process leading to the formation of thrombin and, ultimately, of fibrin. Clinical data indicate an association of JAK2V617F mutation with the severity of the disease. Biological data also show an association of this mutation with the expression of cellular and soluble biomarkers of clotting system activation.
Future preclinical research should focus on the pathophysiology of thrombosis in ET and PV, particularly in terms of the relationship between dys-regulated JAK2 and abnormalities in blood cell count and phenotypical composition. A better understanding of the molecular events leading to the development of ET and PV may provide appropriate targets for the production of bifunctional therapies, i.e., capable to attack the malignant process, as well as to reverse the coagulopathy.