The nucleosome is the basic repeating subunit of chromatin (1), which has a regulatory role in gene transcription as well as serving to package DNA. It consists of about 166 bp of DNA wound in two left-handed superhelical turns around a wedge-shaped histone octamer (a tetramer, H32H42, of histones H3 and H4, flanked by two H2A.H2B dimers), a length of linker DNA which may vary from effectively zero (yeast, mammalian cortical neurons) to 74 bp (sea urchin sperm) and defining the repeat length of the chromatin (~170 to 40 bp), and one molecule of linker histone (H1 or one of its variants; see Histones). Nucleosomes are responsible for the familiar beaded appearance of chromatin seen in the electron microscope. The nucleosome is a wedge-shaped disk, about 110 A in diameter and about 55 A high, with a pseudo-dyad axis of symmetry close to which the entering and exiting DNA duplexes lie.
Nucleosomes (and runs of two, three, etc, nucleosomes) may be excised from chromatin by cleavage in the more accessible linker DNA using, for example, the double-stranded endonuclease micrococcal nuclease (Staphylococcal nuclease) and then fractionated by sedimentation velocity centrifugation through sucrose gradients. Further digestion of mononucleosomes with micrococcal nuclease, which has exonuclease as well as endonuclease activity, proceeds in two stages. First, the DNA is trimmed to ~166bp, at which point histone H1 presents a barrier to further digestion and a metastable particle termed the "chromatosome" is produced. Further digestion of the chromatosome trims the DNA further, causing loss of histone H1, presumably as its binding site close to the nucleosome core is invaded, and then trims the DNA back to 146 bp, where contacts with the octamer block further digestion. The limit product of digestion is the nucleosome core particle whose structure is now known at high resolution (see text below). Protection by histone H1 in the chromatosome was believed to be symmetric, with 10 bp protected at each end of the core particle. It now appears, however, that it may be asymmetric (2, 3), with 20 bp protected at one end of the core particle and none at the other (assuming that the addition of linker histone did not shift the dyad by one helical turn).
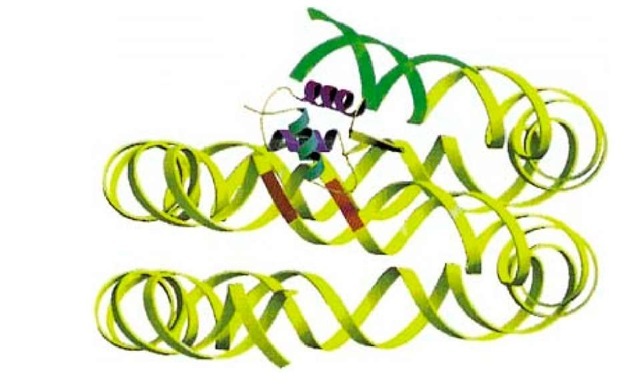
The exact location of H1 in the nucleosome has been controversial (summarized in Ref. 4), because there is not yet any direct structural information for a chromatosome. The general view from several lines of evidence was that the globular domain of H1 bound close to, or over, the dyad axis, on the outside of the DNA gyres, and that it contacted two duplexes through two DNA binding sites (see Histones) that were shown (5) to be required for correct binding to two duplexes and to chromatin. However, results obtained for a nucleosome reconstituted on to Xenopus 5S rRNA gene, which contains a strong nucleosome positioning sequence (see Chromatin), suggested a fundamentally different picture, with the globular domain bound away from the dyad axis and inside the DNA gyres, contacting only one duplex (6). It may be that this is a unique feature of the 5S nucleosome, although this would be a little surprising. Current evidence (summarized in Ref. 7) suggests that, for at least the majority of chromatosomes (a bulk population from chicken erythrocytes), the globular domain of H5 makes two DNA contacts, one of which is indeed near the dyad. Helix III, the "recognition helix" in the globular domain (by analogy with the homologous structures of HNF3g and cyclic AMP receptor protein; see Histones), contacts one of the exiting/entering duplexes, about one helical turn from the end of the chromatosomal DNA, and the second site makes a contact that is in the vicinity of the dyad (Fig. 1). This location is such that the basic C-terminal domain of H1 would be directed toward the linker; a linker location of this domain is suggested by electron microscopy of mononuclesomes (8). It has been argued (7) that the globular domain could well be binding to the 5S nucleosome in the same way, if assumptions about the position of the octamer in that system were to prove to be incorrect; however, the possibility remains that the 5S nucleosome is different.
Figure 1. The location of the globular domain of histone H5 in the 166-bp chromatosome. The recognition helix (magenta) makes a contact one double-helical turn from the end of the DNA (region shown in green) and the second binding site on the opposite face of the domain contacts the central gyre, close to the pseudodyad (3 bp around the dyad shown in red).
1. Nucleosome Core Particle
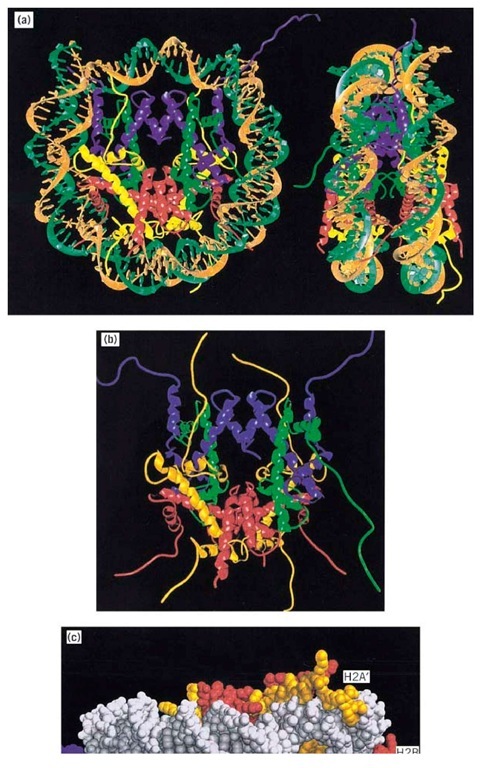
The length of DNA in nucleosome core particles produced by limit nuclease digestion as described above is relatively homogeneous, allowing particles with sequences representing essentially the entire genome to be crystallized (despite the mixed DNA sequences, histone variants, and post-translational modifications). The year 1985 saw the publication of an X-ray crystallography structure of the 206,000 Da nucleosome core particle to 7 A resolution, which confirmed the general shape and arrangement of histones and DNA inferred from earlier studies using electron microscopy and single-particle reconstruction analysis. Determination of the structure of the histone octamer (free of DNA) to 3.1 A resolution in 1991 (see Histones) allowed some of the details of protein-DNA interactions to be inferred, but the full picture has become available only in the 2.8 A structure of the nucleosome core particle (9) published in 1997 (Fig. 2). To obtain this resolution, highly homogeneous nucleosome core particles were reconstituted (see Chromatin) from synthetic 146-bp palindromic DNA and homogeneous recombinant histones produced in Escherichia coli. This is also the largest piece of DNA whose structure has been determined.
Figure 2. Nucleosome core particle structure at 2.8 A resolution. (a) Left: A view down the superhelical axis; right, view perpendicular to the superhelical axis. The pseudo-twofold axis is aligned vertically with the DNA center at the top in each case. The DNA is shown in brown and turquoise; H3, H4, H2A, and H2B are shown in blue, green, yellow, and red, respectively. (b) The histone octamer structure in the nucleosome core, formed by removing the DNA from the nucleosome core particle image in (a). (c) Histone tails between DNA gyres shown in a space-filling model.
The DNA in the core particle is wound in 1.65 superhelical turns on a left-handed helical ramp on the octamer surface; the remaining 10 bp at each end are essentially straight due to contacts between neighboring molecules in the crystal, to give pseudocontinuous double helices. The core particle has a pseudodyad axis of symmetry that coincides with the dyad axis of the octamer and passes between the two H3 molecules. One base pair in the 2.1 A structure sits on the dyad, so the DNA of the two "halves" of the particles (73 and 72 bp) are not identical, despite the initial choice of a palindromic sequence in an attempt to obtain a nucleosome core particle with exact two-fold symmetry! The DNA superhelix is not uniformly bent, as was already known, due to the nature of the local histone-DNA interactions, the sharpest distortions being at positions 15 bp and 40 to 50 bp on either side of the dyad (positions±1.5 and ±4 to 5 relative to the dyad as position 0). The overall helical periodicity of the DNA in the core particle is 10.2 bp per turn, but varies in detail along the DNA.
The organization of histones is very similar to that seen in the structure of the histone octamer on its own at 3.1 A resolution (10), with histone folds arranged in handshake motifs (Fig. 2 a). The histone folds organize the central 121 bp of DNA; the remaining 13 bp at each end are organized by N-terminal a-helical extensions to the folds in the two H3 molecules and adjacent residues from the N-terminal tail domain. Each histone-fold dimer in the octamer organizes 27 to 28 bp, with 4-bp stretches between contacts. Each dimer has three independent DNA-binding sites: two contributed by the loops at each end of the dimer, and one formed by the two N-termini of a-helices of each histone fold at the dimer apex. Histone-DNA contacts include extensive salt bridges and hydrogen bonds from both main chains and side chains of the histones to the phosphate backbone, together with nonpolar contacts with DNA sugar groups, and electrostatic interactions of the positively charged N-termini of a-helices in the histone folds with DNA phosphate groups. Such interactions might be expected for proteins that can bind to a variety of DNA sequences; the number of specific base contacts in the core particle structure is very small (one of them being a nonpolar contact of the 5-methyl group of a thymidine in the major groove). Strikingly, arginine side chains contact the minor groove each of the 14 times it faces inwards.
A surprising finding was that the N-terminal tails (see Histones), which are the most variable regions between species and which are the sites of post-translational modification, notably acetylation (see Histone Acetylation), are directed outward from the core particle, exiting between the DNA gyres; they are therefore well-placed to make contacts with other nucleosomes or other proteins. Some of the tails are visible in Figure 2a; Figure 2b shows the DNA stripped away to show the extended histone tails and the histone folds in the octamer. The four H3 and H2B tails exit through the narrow channels formed by perfectly aligned minor grooves of adjacent gyres of the DNA superhelix (Fig. 2c), the alignment being a consequence both of the change in the twist of the DNA from 10.6 bp in solution to 10.2 bp in the core particle and local adjustments to the twist. H4 and H2A tails exit in minor grooves either on the "top" or the "bottom" of the core particle. The accessible histone tails in chromatin are known to be targets for transcription-linked histone acetyltransferases (see Histone Acetylation) as well as recognition sites for proteins that lead to a more repressive chromatin structure (eg, SIR proteins, giving silenced regions at yeast telomeres and mating type loci) and for at least one chromatin remodeling complex (NURF) (see Chromatin). Histones are thus not simply packaging proteins but also have a gene regulatory function. DNA could be weakly bound to the outside of the core particle (but with low occupancy; otherwise they would have been seen in the core particle structure), and acetylation could well prevent this. Such a weak effect alone is unlikely, however, to account for the enhancing effect of acetylation on transcription in vivo, although it could perhaps account for results of some in vitro assays in which acetylation can increase the access of transcription factors to mononucleosome DNA (see Histone Acetylation). The nature of the crystal contacts hints at possible roles for the tails. The basic H4 tail, which has been implicated in a variety of biological functions, contacts an acidic patch on the surface of a neighboring octamer. Residues 16 to 25 of H4 (the most internal/proximal acetylation site is at position 16) make multiple interactions with acidic side chains on H2A and H2B in an adjacent nucleosome in the crystal. Model building shows that a similar interaction could occur in the solenoid model for chromatin higher-order structure (9) (see Chromatin). Acetylation might then result in disruption of higher-order structure, with the relaxed structures being more accessible to transcription factors, remodeling complexes, and the transcriptional apparatus (see Chromatin). Residues 16 to 24 of H4 are also known to be necessary for interaction with Sir3 in yeast silencing and heterochromatin formation, and in this case the suggestion is that the internucleosomal contact is replaced by an interaction with Sir3 (9).