Luciferase is the generic term for an enzyme that catalyzes a reaction that produces light; luciferin is the general term for a substrate of these reactions. Luciferin and luciferase are both named from the Latin lucifer, referring to Venus, the morning star. (Lucifer literally means light-bringing.) Luciferases all catalyze oxidative reactions that give off light (and as such require oxygen), but other than this, different luciferases may have little or nothing in common. There are many different bioluminescent systems in nature, and consequently many different luciferins and luciferases.
In general, the reactions catalyzed by luciferases are special cases of chemiluminescent reactions. The general reaction scheme is as follows:
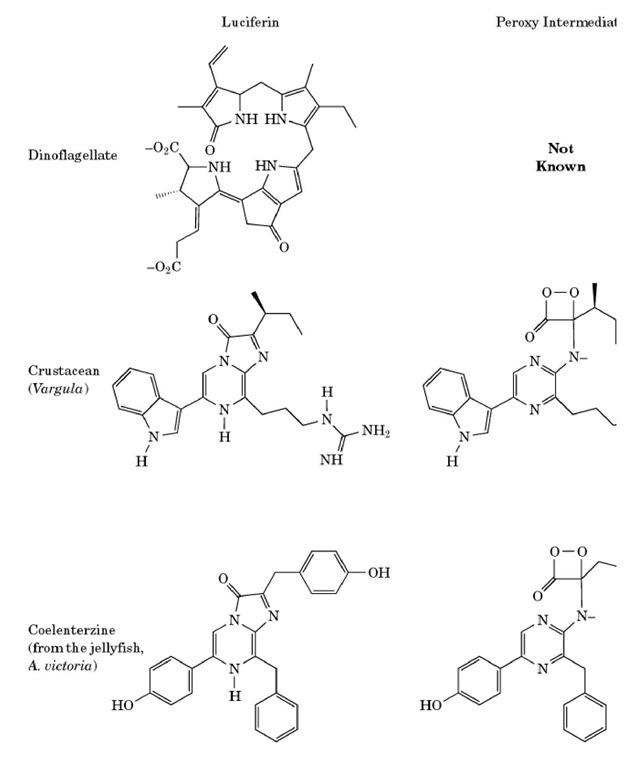
Thus, luciferases catalyze the oxidation of their respective luciferins, producing an excited-state molecule of oxyluciferin (the asterisk denotes an excited state). It is the decay of this molecule to the ground state that is the source of the light. Differences in luciferin molecules, or changes in the environment of the active site, may result in differences in the color of the emitted light. The variety of luciferin structures is demonstrated by the examples shown in Figure 1.
Figure 1. The various forms of luciferin, with the corresponding peroxy intermediate and oxyluciferin.
1. Firefly Luciferase
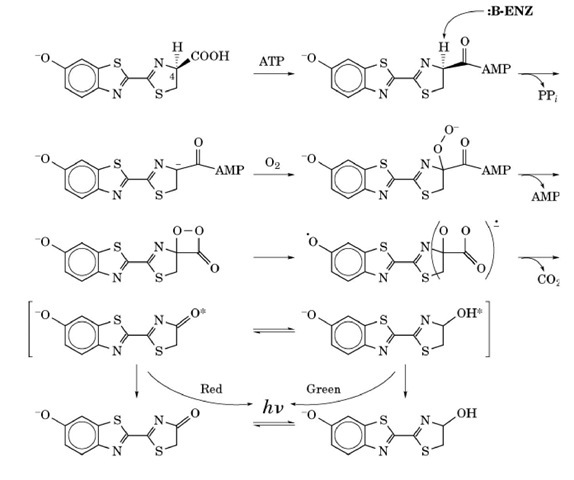
Perhaps the most widely known bioluminescent reaction is that of the common firefly. This reaction is exceptional in that it has a high quantum yield of about 90%, meaning that about 90% of the reacting molecules will emit a photon of light. The detailed mechanism of the firefly reaction is shown in Figure 2. In the first step of the reaction, the carboxylic acid moiety of the firefly luciferin is activated by reaction with ATP to produce an adenylated intermediate. The C4 proton is abstracted, producing a carbanionic form of luciferin. This is the form of luciferin that reacts with molecular oxygen to produce the key intermediate, an activated dioxetanone. This dioxetanone is unstable because of the high strain energy in the four-membered ring and because of the inherently weak peroxide bond. The breakdown of this intermediate is generally believed to occur by a chemically induced electron exchange luminescence (CIEEL) mechanism (discussed below) and is accompanied by loss of carbon dioxide. The final product is the excited state of oxyluciferin, which can exist in either the keto or the enol forms of excited oxyluciferin, which are responsible for emitting red and yellow-green light, respectively.
Figure 2. Mechanism of bioluminescence in the firefly.
The generation of the excited state is thought to proceed by the CIEEL mechanism. This hypothesis states that the benzathiazole portion of the molecule acts as an electron-rich (easily oxidizable) donor molecule that donates an electron (going from negative to neutral in the process) to the peroxide moiety, which facilitates cleavage of the O-O peroxide bond, leaving one oxygen atom with a full negative charge, and one as a neutral radical. After loss of the carbon dioxide, the electron is returned to the donating moiety, yielding excited-state oxyluciferin.
The firefly is not the only species to make use of a dioxetanone intermediate. Other organisms such as the sea pansy Renilla reformis, the marine crustacean Cyprdina hilgendorfii, and the jellyfish Aequorea victoria have luciferins of markedly different structures, but each is thought to proceed through a dioxetanone intermediate. The color of light emitted by these species also differs from that of the firefly reaction. It is important to note, however, that the color of light emitted is not determined solely by the structure of the luciferin. The enzyme and its microenvironment also have an important role to play. It is possible to generate mutant forms of luciferase in the laboratory that emit light of a different color than the naturally occurring reaction.
Although the reaction mechanism for firefly luciferin is known in some detail, and the structure for firefly luciferase has recently been solved (without bound luciferin), the details of the interaction between the luciferin and luciferase are not known. However, based on sequence analysis of luciferases from many firefly species, the active site of firefly luciferase has been tentatively identified. The structure is a "hammer and anvil" structure, where there is a small C-terminal "hammer" domain atop a large ^-terminal "anvil" domain. Many of the putative active-site residues are at the interface between these domains. It is speculated that since, like all luciferase reactions, water must be excluded in order for the bioluminescence reaction to occur with a high photon yield, the hammer and anvil regions of luciferase clamp together, with the substrates in between and excluding water in the process. Of course, the enzyme must also provide general bases for abstracting protons involved in the mechanism, but no specific amino acid residues have yet been implicated.
2. Bacterial Luciferase
One of the best characterized bioluminescence systems is that from bacteria. Several different species of luminescent bacteria exist, but the luciferases from the various organisms are homologous. Unlike most bioluminescent systems, however, the bacterial luciferase system does not have a luciferin molecule that is unique to the bioluminescence reaction. Instead, these systems use reduced flavin mononucleotide (FMNH 2) and a long-chain fatty aldehyde, which are converted to oxidized flavin and the corresponding carboxylic acid. Similar to other bioluminescent reactions, this oxidation reaction requires the use of molecular oxygen. Another important difference between this reaction and most bioluminescent reactions is that the peroxide species leading to the light emission is a linear rather than a cyclic peroxide. However, like firefly luciferase, the structural details of the interaction of bacterial luciferase with its substrates are not well understood. The overall chemical reaction is as follows:
where R-CHO is a long-chain aldehyde, R-COOH is the corresponding carboxylic acid, and FMN is oxidized flavin mononucleotide.
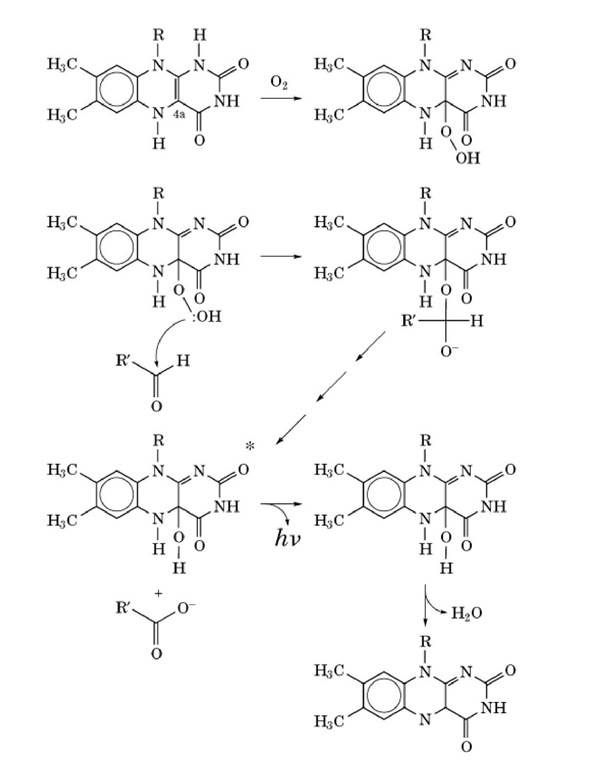
The reactants must bind to the luciferase in a specific sequence; FMNH2 first, then O2, then the aldehyde. A detailed scheme of the bacterial bioluminescence reaction is shown in Figure 3. The formation of a 4a-peroxyflavin from FMNH2 and O 2 is the first step in the reaction. This peroxyflavin may then react with the aldehyde to form a tetrahedral intermediate (the formation of an alkyl-flavin peroxide bond should be noted). Breakdown of the tetrahedral intermediate involves cleavage of the peroxide bond along with the formation of an excited-state 4a-hydroxyflavin and a carboxylic acid. The hydroxyflavin emits light as it relaxes to the ground state and then undergoes dehydration to yield FMN and H2O.
Figure 3. Mechanism of bioluminescence in bacteria.
Bacterial luciferase is a heterodimer, consisting of two homologous a and b subunits with a total molecular weight of about 76 kDa. The two subunits have an overall sequence identity of about 40%, and a similarity of about 80%. The crystal structure of the enzyme in the absence of substrates has recently been solved. The two subunits each form a TIM barrel structure, with a four-helix bundle as the core of the interface between the subunits. Both subunits are required for normal activity, but there appears to be only one active site per dimer. There is a narrow channel leading into a large hydrophobic cavity in the core of the a subunit, and this is believed to be the active site of the enzyme. Cys 106 is located at the mouth of this cavity; modifications to this residue have been shown to compromise the catalytic activity of the enzyme greatly. Further, two tryptophan residues, 194 and 250, which are postulated to be near the flavin binding site on the basis of spectroscopic measurements, are found lining this cavity. Mutation of either of these tryptophans reduces the FMNH2 binding ability of the enzyme.
In the present crystal structure, residues 262-290 are unresolved, which strongly suggests that this segment of the polypeptide chain is flexible. Given the structure of the rest of the enzyme, it is likely that the unresolved amino acids form a "lid" that covers the entrance to the active site. It is known from proteolysis and chemical modification experiments that a conformational change occurs during the catalytic cycle of the enzyme. It is speculated that this conformational change may be the flexible lid closing over the active site after substrates have been bound, excluding water from the reaction. Another interesting feature of the internal cavity centers around Asp 113. In the crystal structure, this residue forms a hydrogen bond network with His 44 and His 45, which mutational analysis have also shown to be important for activity and flavin binding. Although Asp 113 is not itself part of the putative active site cavity, His 44 and 45 are. Substitution at position 113 with neutral or positively-charged amino acids results in activity loss of three to six orders of magnitude. It is clear that disruption of this hydrogen bonding network interferes with the activity of the enzyme. Although the reason for this is not yet understood, it is believed to be due to distortion of the active site.
3. Aequorin and Calcium-Binding Photoproteins
Calcium binding proteins constitute another category of luciferases. The best known of these is aequorin from the jellyfish Aequorea victoria. Aequorin was originally thought to be an exception to the general rule that all bioluminescent reactions require molecular oxygen or hydrogen peroxide, because isolates of the enzyme would emit light on addition of calcium, even under anaerobic conditions. However, it was eventually determined that the cofactor for aequorin, called coelenterazine, is covalently bound to the enzyme in a reaction requiring molecular oxygen. Thus, once the coelenterazine is bound to the enzyme, it is activated and needs only calcium for the bioluminescence reaction to occur. Once the calcium is added, aequorin undergoes a conformational change and becomes capable of catalyzing the oxidation of the bound coelenterazine. The products of the reaction are light, carbon dioxide, and apoaequorin with noncovalently bound coelenteramide. Reconstitution of the activated enzyme requires molecular oxygen and fresh coelenterazine in reducing conditions. The mechanism of the bioluminescence reaction in aequorin is under some debate. It seems clear, however, that the mechanism has similarities to the firefly reaction, including a dioxetanone-like intermediate and CIEEL-type charge transfer.
4. Green Fluorescent Protein
Aequorea (and some other bioluminescent coelenterates) have a second protein in their bioluminescent systems. This noncatalytic protein is the green fluorescent protein (GFP). GFP absorbs maximally at 395 nm and has a smaller absorption peak at 470 nm, while the bioluminescence emission spectrum of aequorin has a maximum at 470 nm. Thus GFP is able to act as an antenna protein and to accept the energy from the bioluminescent reaction of aequorin. GFP then emits light with a maximum intensity at 509 nm. The presence of GFP serves to increase the quantum yield of the aequorin. The crystal structure of GFP has recently been solved; it is an 11-stranded b-barrel surrounding a central a-helix, capped by three smaller distorted helices. The chromophore for GFP is a modified hexapeptide derived from the sequence Phe-Ser-Tyr-Gly-Val-Gln, which is found in the interior of the b-barrel.
5. Applications of Luciferase Technology
Although the study of luciferases is a fascinating academic pursuit in its own right, luciferase research has yielded many technological benefits as well. Bioluminescence is experimentally a very useful characteristic for an enzyme to have, because light production can be detected at extremely low levels and over a wide range of intensities. This, coupled with the fact that bioluminescence assays are quite rapid, has made luciferases the assay of choice in many different systems. Firefly luciferase is sold commercially as a sensitive and accurate assay for ATP, and it is able to detect ATP down to femtomolar (10-15 M) concentrations. Similarly, aequorin may be used as an equally sensitive assay for calcium. Finally, bacterial and firefly luciferases are commonly used as markers for genetic engineering, in the study of gene regulation, and for many other laboratory and industrial uses. Luciferases are singularly useful in these contexts, as they allow for sensitive reporting of molecular events as they occur within living systems.
![tmp14F-99_thumb[1] tmp14F-99_thumb[1]](http://what-when-how.com/wp-content/uploads/2011/05/tmp14F99_thumb1_thumb.jpg)