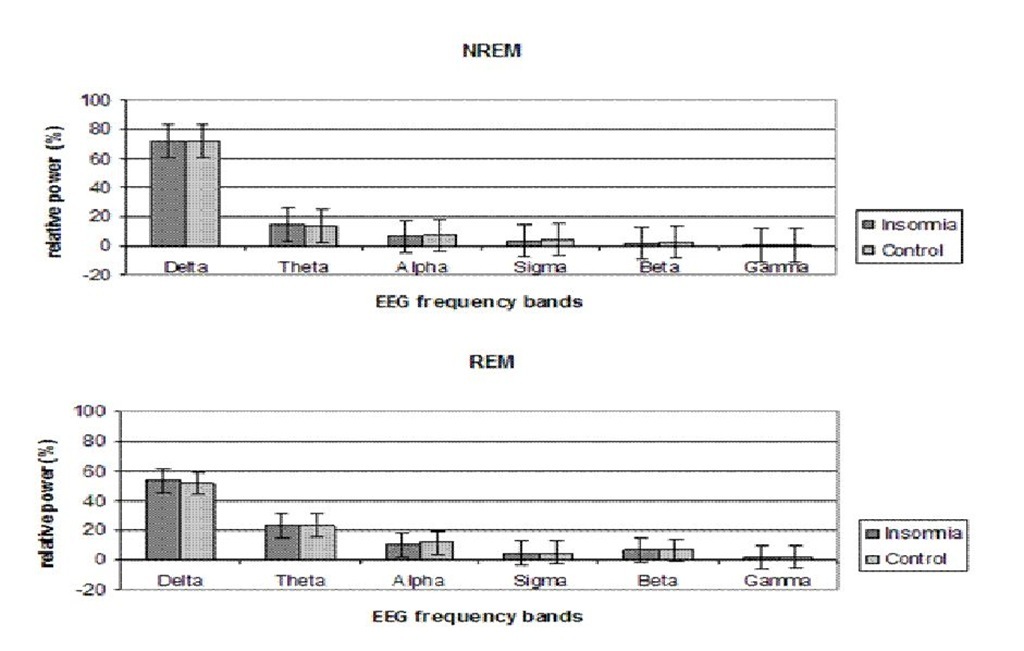
The Sleep EEG: NREM/REM
First, the average of all the previously defined relative frequency bands during NREM and REM sleep of the entire night was calculated (figure 5). No significant differences were found between both groups.
Figure 5. Relative spectral power profiles during NREM and REM sleep. An average of the entire night was calculated for each frequency band. Error bars denote standard error.
Since the number of sleep cycles was not equal for all participants, an average of NREM and REM sleep was calculated for the first three sleep cycles. Again, no significant differences were found between insomnia patients and healthy sleepers.
Furthermore, an assessment was made of the evolution of all described EEG frequencies from the first to the third NREM and REM cycle, but no significant differences were found.
Correlational Analysis of Arousal and Sleep Parameters
As previous studies have shown that arousal and sleep disruptions are interrelated, a correlational analysis was performed to evaluate possible links between objective and subjective sleep parameters and arousal components. Given the risk for multiple comparisons and chance hits, only the following correlations were analyzed: objective SOL and arousal components, discrepancy between objective and subjective SOL and arousal components, and cortisol and sleep fragmentation parameters. More specific it was hypothesized that specific sleep EEG frequencies are related to objective sleep disruption and sleep misperception: delta and beta EEG activity during the SOP and NREM sleep were compared to the arousal index and TST discrepancy.
Objective Sleep Parameters and Arousal
A Spearman Rank correlational analysis was performed for each group to evaluate the possible associations between objective sleep parameters and arousal components. The objective SOL was negatively correlated with the average absolute delta![]() > EEG activity during the SOP in insomnia patients. Evening cortisol level was positively correlated with the arousal index
> EEG activity during the SOP in insomnia patients. Evening cortisol level was positively correlated with the arousal index![]() and number of awakenings
and number of awakenings![]() during the night. Relative
during the night. Relative
beta EEG activity during NREM sleep was positively correlated with WASO![]() and the arousal index
and the arousal index![]() , and negatively correlated with TST
, and negatively correlated with TST![]() .
.
Relative beta power during REM sleep was also positively correlated with the arousal index
Within our group of healthy sleepers, the objective SOL was negatively correlated with absolute delta power![]() Furthermore, a positive correlation was found between cortisol and PSAS-SOM
Furthermore, a positive correlation was found between cortisol and PSAS-SOM![]() Relative beta EEG activity during NREM sleep was positively correlated with
Relative beta EEG activity during NREM sleep was positively correlated with![]() number of awakenings
number of awakenings![]() , and the discrepancy of TST
, and the discrepancy of TST ![]()
The only correlation observed in both groups, as well as in the total sample of subjects (insomnia and controls as one group) was the negative correlation between objective SOL and the amount of absolute delta power![]()
Subjective Sleep Parameters and Arousal
A Spearman Rank correlational analysis was performed for each group to evaluate the possible associations between subjective sleep parameters and arousal components.
The SOL discrepancy in insomnia patients was positively correlated with the PSAS-SOM ![]() and cortisol level
and cortisol level![]()
The SOL discrepancy in the control group was positively correlated with the EMG level ![]() during the SOP. Relative beta EEG activity during NREM sleep was positively correlated with the discrepancy of TST
during the SOP. Relative beta EEG activity during NREM sleep was positively correlated with the discrepancy of TST![]()
Conclusion
Clinical Data
In this study, a relatively homogenous group of ‘objective’ insomnia patients was targeted and compared to a matched control group of good sleepers. Indeed, our insomniacs were clearly characterized by sleep disruption as reflected by the PSG data. They tended to take more time to fall asleep, were more awake during the night, had less TST and a decreased SE. Moreover, both the macro- and microstructure of sleep were significantly fragmented, reflected by more awakenings and a higher arousal index in comparison to controls. Interestingly, when evaluating the effect sizes for all objective sleep parameters, the highest effect was found for the arousal index, which is a strong argument for including this parameter in standard sleep evaluation. An often reported phenomenon in insomnia patients is the fact that perception of sleep quality is not in accordance with objective findings, a result also present in our insomnia group. In line with former research [51-53, 56], our group of insomniacs—although characterized by objective sleep disruption—tended to overestimate SOL and WASO, as well as underestimate TST and SE. The discrepancy between perception and polysomnography has been related to the occurrence of arousals [89]; however, our results did not show a positive correlation between the arousal index and perception of sleep within the insomnia group, suggesting that another factor might be in play.
The insomnia patients were also characterized by higher levels of trait and state anxiety, as well as more cognitive arousal instead of physiological arousal, a finding previously reported by Lichstein and Rosenthal [3]. Furthermore, trait anxiety was related to the perception of the SOL, while this was not the case in the control group. Moreover, there appeared to be a relationship between state anxiety and presleep arousal in insomnia patients. Tang and Harvey [76] showed in a napping experiment that the presence of both anxiety and cognitive arousal have a greater impact on sleep and perception of sleep. Indeed, in their study only the anxious cognitive arousal group showed a greater discrepancy in TST, as opposed to a neutral cognitive arousal group in comparison to a no manipulation group.
Arousal in Insomnia: A Dynamic and Transitional Concept?
Our results confirm the presence of arousal in insomnia patients. First of all, the heightened scores on the trait anxiety subscale of the STAI questionnaire suggests that our insomnia patients were characterized by a predisposition to react with an emotional arousal response in stressful situations, which corresponds with the behavioral perspective and neurocognitive model of insomnia. Furthermore, the results of the STAI-2 subscale confirm the presence of anxiety in stressful situations. During the presleep period, they also reported more cognitive arousal, a result in line with previous studies [27, 28, 30, 42]. As in the study of Lichstein and Rosenthal [3], presleep physiological arousal was also absent in our study sample. As discussed previously, research regarding the presence of physiological arousal reflected by EMG levels or cortisol show mixed results. Besides the subjective physiological arousal, muscle tension during the SOP and evening cortisol were measured to evaluate the objective physiological arousal related to the sleep environment and sleep time, but no effects were found. This corresponds to the studies performed by Riemann et al. [25], as well as Varkevisser et al. [26], who also found no indication of physiological arousal whatsoever. As the insomnia group also failed to report a significant increased physiological presleep arousal over the last month during the screening period, it could be suggested that this was just a characteristic of this patient sample.
Next, an indication was found for an impairment of cortical sleep initiation processes during the SOP. The absence of a decrease in alpha power in combination with the slower increase in delta power, suggests that normal sleep onset processes were delayed, resulting in longer sleep onset latencies. This result is in agreement with previous studies evaluating the sleep onset period [60, 61]. We did not find, however, any significant differences for beta EEG activity, both during wakefulness and sleep. Again, these results emphasize the fact that hyperarousal in all three systems studied is apparently not always simultaneously present in insomnia. Arousal most probably should not be seen as a static phenomenon.
When reviewing the process of falling asleep at the level of EEG changes, we see that two shifts need to occur before sleep onset is achieved. First, high frequency EEG activity needs to decrease, and secondly low frequency EEG activity needs to increase [58, 59]. According to the neuronal group theory [64, 90] maximal alertness and maximal sleepiness are part of a continuum, suggesting that transitional states are possible as well. Furthermore, the Neuronal Transition Probability model [65] posits that oscillatory modes of the thalamocortical neurons responsible for the beta, sigma and delta oscillations clearly show this transitional character of arousal and sleepiness. Therefore, it might be suggested that maximal alertness is equal to hyperarousal, reflected by both increased levels of beta EEG activity and decreased levels of slow wave activity in the delta range. A second phase could be referred to as arousal; it is a condition in which hyperarousal is not present anymore, but complete de-arousal is still not reached, reflected by normal levels of beta EEG activity and slower increase of delta power. Finally, when complete de-arousal is reached, a normal transition from wake to sleep can occur. In light of this perspective, it can be assumed that our insomnia patients were characterized by a lack of complete de-arousal, reflected by impairment in sleep initiation processes as seen in the slower EEG frequencies during the SOP. The strong correlation found between the sleep onset latency and the amount of delta power both in healthy sleepers and insomnia patients strengthens the hypothesis that the depolarization to the delta mode of the TC neurons, and thus the speed of transition from wake to sleep is the key factor, and not the beta EEG power. This perspective can also be used to explain the lack of hyperarousal during NREM and REM sleep. If, according to this theory, our insomniacs were not characterized by hyperarousal, but by a lack of complete de-arousal, heightened levels of beta EEG activity were not expected. Moreover, the frequent awakenings and arousals may be an indication of a certain degree of instability of the sleep system because of the arousal condition, since these intrusions are defined by the presence of higher frequencies such as alpha or beta EEG activity. When arousal is considered as a varying construct on a continuum, the mixed results regarding hyperarousal in insomnia can be better interpreted. Assuming that the maximal state of hyperarousal is accompanied by increased levels on all 3 components of the multidimensional arousal construct, the gradual decrease in hyperarousal will lead to a normalization of some arousal components. For example, our insomnia patient sample apparently was only characterized by presleep cognitive arousal, state anxiety, impairment of sleep initiation processes and instability of the sleep maintenance processes. As such, it could be hypothesized that physiological and cortical hyperarousal will be more apparent when reaching the upper level (hyperarousal) of the continuum, while cognitive arousal is a sign of a minimal arousal level. Indeed, cognitive arousal is much more prevalent in insomnia studies than physiological arousal [3] and is often regarded as the starting point for the development and maintenance of insomnia and related arousal changes [32, 35].
Of course, the recruitment of our patient sample on the basis of their polysomnographical sleep distortions might have influenced the cortical arousal data, given the fact that Krystal and coworkers [68] found that specific EEG correlates of cortical arousal were more pronounced within a group of ‘subjective’ insomnia patients in comparison to ‘objective’ insomniacs and controls. However, our insomnia patient group showed a high degree of underestimation of TST (19.5%), but this was not accompanied by increased high frequency EEG activity, as would be expected by the theory posited by Krystal and colleagues. Furthermore, Buysse et al. [69] found that only women are characterized by heightened levels of beta EEG activity during sleep. Given that our insomnia and control groups consisted of more men than women, it is possible that cortical arousal reflected by beta EEG activity was not a predominant feature.
Sleep and Arousal
Based on these results and recent literature, we propose to conceptualize arousal as a multidimensional construct, expressing itself in different systems with varying degrees. To what extent is arousal associated with the specific sleep impairments, both objectively and subjectively? Previous studies have found different correlations between arousal and sleep disruption, again pointing out the heterogeneity of this construct and its role in sleep. On the one hand, we found a correlation between evening cortisol and number of awakenings and arousal index, a finding in accordance with Vgontzas et al. (1998, 2001) and Rodenbeck et al. (2002). Also, cortisol levels were associated with the misperception of the SOL. These results suggest that physiological arousal reflected by evening cortisol is associated with a significant fragmentation of sleep macro- and microstructure, as well as a misperception of the time needed to fall asleep. On the other hand, the subjective physiological presleep arousal component seemed to be related with the discrepancy of SOL. Although our insomnia patients were not characterized by increased levels of beta EEG power during wakefulness and sleep, correlations have been found with objective sleep parameters. It seems that the presence of beta EEG power, both during NREM and REM sleep, is related to sleep fragmentation, reflected by the arousal index. An important remark related to this issue is the fact that all awakenings and arousals were excluded from spectral analysis, which means that this correlation can not be due to the mere presence of arousals in insomnia patients. Furthermore, NREM beta EEG activity was also correlated with objective WASO, TST and SE. This seems not to be in accordance with previous research suggesting that beta EEG activity is specifically related to subjective insomnia and the perception of sleep [66-68]. Our insomnia sample seemed to be characterized by a discrepancy of sleep perception, in combination with a lack of cortical hyperarousal reflected by high frequency EEG activity. Could it be that this was a specific subtype of arousal-related insomnia, in which the beta EEG activity is more associated with objective PSG disruption both at the level of macro-and microstructure? Misperception of TST was associated with the amount of alpha EEG power during REM sleep, a result not mentioned before. Furthermore, misperception of SOL in our study was related to the somatic component of the PSAS, and not the cognitive arousal component as was previously shown by Tang and Harvey [76]. The fact that they experimentally induced arousal in healthy sleepers might be an important factor. However, De Valck et al. [75] did not find any correlations between the experimentally induced arousal and increased sleep onset latencies measured by an MSLT. Could it be due to individual differences in predisposing factors?
In summary, the insomnia group was characterized by more cognitive presleep arousal, but no association was found with any sleep variable. Instead, the physiological component of arousal appeared to play an important role in influencing both objective and subjective sleep, reflected by the correlation between cortisol and sleep fragmentation and somatic presleep arousal and perception.
Conclusion
In this study we used a relatively homogenous sample of insomnia patients characterized by objective sleep distortions as shown by a polysomnography. They reported sleep maintenance problems in combination with sleep onset difficulties with a dominance of the first type. In accordance with previous studies evaluating NREM/REM EEG profiles in insomnia patients [66, 67], analyses were performed on the first PSG recording.
Summarizing, this group experienced more cognitive and emotional arousal, but no increase in physiological arousal, both subjectively as well as objectively, in comparison with a control group. Indications of cortical arousal were only present during the sleep onset period, reflected by a stable alpha EEG level and slower increase of delta power, resulting in longer sleep onset latencies. Correlational analysis on the other hand, revealed a stronger association between physiological parameters of arousal with sleep, in comparison to the cognitive arousal component. Furthermore, the cortical arousal variables were correlated with objective sleep disruption, not with sleep perception. Together with literature, these results point to a large variability in insomnia patients as to the expression of hyperarousal and the different arousal components. It is suggested to regard hyperarousal as a more transitional state that can vary on a continuum going from maximal alertness/hyperarousal to maximal sleepiness/de-arousal. Future research should take into account the possible variation within the insomnia population of this construct and the role of specific predisposing factors, and examine the specific effects of different degrees of hyperarousal through experimentally induced arousal studies as to further clarify the role of this concept in insomnia and its different subtypes.