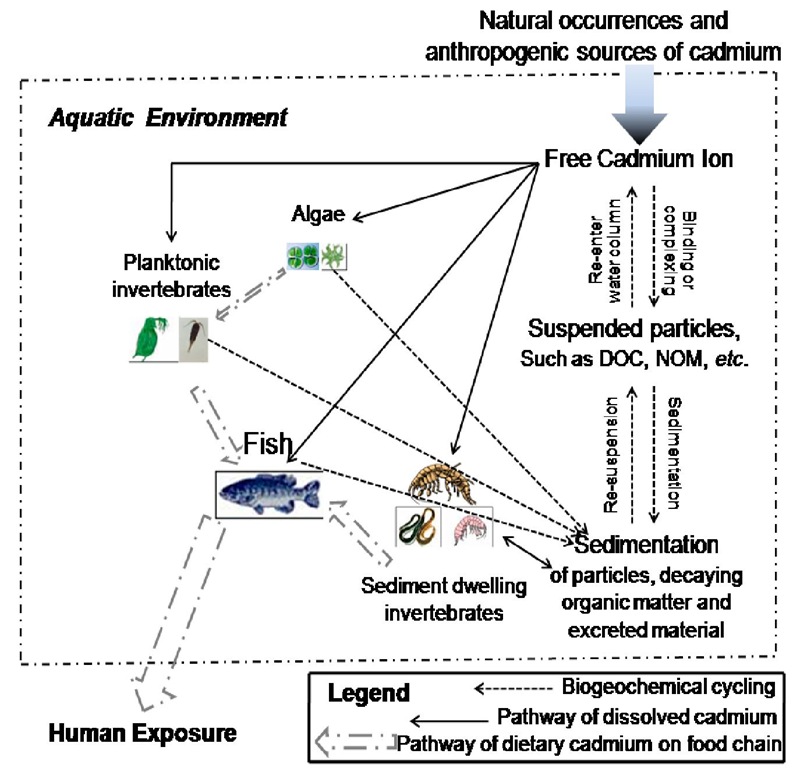
Cadmium is a relatively rare element that is a minor nutrient for aquatic organisms at low concentrations, but is toxic to aquatic biota at concentrations only slightly higher. In aquatic environments, organisms are exposed to cadmium in dissolved and particulate-bound forms, including ambient water, sediments, and food (Figure 7).
Cadmium can be taken up by bacteria, phytoplankton, zooplankton and fish directly or through the food chain as a potentially toxic metal, which can enter the organism body via waterborne and dietary pathways, although regulatory assessments of metal toxicity to aquatic organisms assume that toxic effects are caused by dissolved metals.
In recent years, concern has been expressed about the possible effects on aquatic organisms from exposure to cadmium. Both acute and chronic toxicity tests are essential for better understanding of the response of aquatic organisms to cadmium. This topic provides many different studies on cadmium toxicity to aquatic biota.
Figure 7. The possible routes of aquatic organism exposure to cadmium (direct uptake from water phase or through food uptake).
Acute Toxicity
The acute toxicity of cadmium to saltwater organisms has been determined for 48 species of invertebrates and 12 species of fish, representing the required different taxonomic families (Figure 8). Having data for a variety of organisms is helpful, because different test organisms provide different types of information about the toxicity of cadmium. The range of species tested not only included marine Arthropoda, Crustacea ranging from copepods (Acartia tonsa) to amphipod (Corophium insidiosum), to mysid (Americamysis bahia), to crabs (Cancer magister), and shrimp (Crangon septemspinosa), but included several Mollusca (bivalve), filter-feeding mussels (Mytilus edulis), the Pacific oyster (Crassostrea gigas), and the deposit-feeding clam (Mya arenaria), as well as the squid (Loligo opalescens), and mud snail (Nassarius obsoletus). It also included Chordata (Osteichthyes), Annelida (Polychaeta), Echinodermata (Echinoidea).
Figure 8. Distribution of acute cadmium toxicity data from various saltwater organisms of different phyla or class; Arrows show how 24- and 48-h LC50s of M. monogolica Daday (Saltwater zooplankton species from China) exposed to cadmium compare and rank with these data (USEPA, 2001).
It is noteworthy that the rank of M. mongolica (an indigenous or native species of China) for species sensitivity in Figure 8 was in the range of 46-47 and 32-33 for 24- and 48-h LC50 values, respectively, which indicates that M. mongolica is moderately sensitive to cadmium stress compared with different phyla or different orders of the same phylum (Arthropoda) and class (Crustacea), including amphipod, copepod, mysid, decapods (shrimp, crab), and isopod.
Data given in Figure 8 also indicate that cadmium is not very toxic over short exposure periods, and the LC50s for a wide range of species are usually in excess of 1 mg Cd/L*. It is most unlikely that these concentrations will occur in the future, even in the most polluted situations of Figure 3.
Sensitivity of Species
Both invertebrate and fish species display a wide range of sensitivities to cadmium in Figure 8. Of the 61 saltwater genera for which acute values are available, The SMAVs for saltwater invertebrate species range from 41.29 ^g/L for a mysid to 135,000 ^g/L for an oligochaete worm. So the most sensitive, Mysid, Americamysis bahia, is 3,270 times more sensitive than the most resistant, Oligochaete worm, Monopylephorus cuticulatus.
The average rank number of different class or species is shown in Table 1, which indicated that saltwater aquatic animals had a broad range of cadmium tolerance* including Anthropoda, Echinodennata , Mollusca, Chordata and Annelida.
Anthropoda, especially copepods, appeared to be the most sensitive in this group (Table 1 and Figure 8), but Annelida were the most resistant to cadmium, and no effects were recorded at concentrations less than 100 ^g Cd/L. Echinodermata also appears to be very resistant to cadmium, and no harmful effects were recorded at concentrations less than 100 ^g Cd/L (UNEP, 1982; USEPA, 2001).
Table 1. Rank number of different class or species based on toxicity according to Figure 8.
Organisms are able to survive in polluted environments of high cadmium concentrations because the processes of detoxification must have been developed.
Chronic Toxicity
Acute cadmium toxicity occurs over a wide range of concentrations depending on the organism, the chemical conditions, and the type of test, as do chronic effects. In attempting to elucidate the actual models of toxic action for cadmium, it is perhaps most instructive to consider initially low-level, long-term (i.e., chronic) studies.
Some researchers conducted a 23-day life-cycle test with the saltwater invertebrate, Mysid, Americamysis bahia at 20 to 28 °C and salinity of 15 to 23 g/kg in three chronic cadmium concentrations. Survival was 10% at 10.6 ^g/L, 84% at the next lower test concentration of 6.4 ^g Cd/L, and 95% in the controls. No unacceptable effects were observed at 6.4 ^g Cd/L, therefore the chronic toxicity limits are 6.4 and 10.6 ^g Cd/L, with a chronic value of 8.24 ^g Cd/L (USEPA, 2001).
The effect of cadmium on the reproduction strategy of saltwater zooplankton, the cladoceran M. monogolica was investigated by Wang et al. (2009a). After a 21-day exposure of the 18 ± 6-h old neonates to 0 (control), 4.1, 12.9, 26.7, 47.1, 67.89, 91.6 and 171.8 ^g Cd/L at 20 ± 0.5 °C, the authors compared the survival, number of neonates per female, first day of reproduction and neonate size of the cadmium exposures to the controls. Cadmium negatively influences both survivorship and fecundity of several genera including Moina monogolica Daday. However, the effect on fecundity may not be with the same magnitude on survivorship. For example, cadmium at 4.1 ^g Cd/L affected the fecundity of M. monogolica but had no effect on the survivorship. The reproductive NOEC (no-observed-effect concentration) of 21-day static-renewal test was 1.11 ^g Cd/L cadmium, and the reproductive LOEC (lowest-observed-effect concentration) was 3.01 ^g Cd/L. The resultant chronic value was 1.78 ^g Cd/L (Wang et al., 2009a).
Zooplankton, particularly rotifers and cladocerans are useful for chronic tests since they are not only sensitive to cadmium stress, but also due to their wide distribution, are easy to maintain under laboratory conditions, their parthenogenetic life cycle ensuring the supply of several individuals with little genetic variability and relatively higher population growth rates. Among several biological variables, population growth characteristics serve as sensitive indicators of toxic stress in zooplankton (Garcia et al., 2004).
Population growth studies are helpful to quantify sublethal effects of toxicants including cadmium to zooplankton because small changes in survivorship and fecundity are eventually summed up in peak abundances and growth rates (Halbach et al., 1984). For this, single species tests are appropriate because they yield information both rapidly and quantitatively to evaluate the direct effects of toxicants which could be extrapolated to natural conditions with some precision.
Bioaccumulation (INTERNAL METAL EXPOSURE)
Cadmium metal is slightly soluble in water, although its chloride and sulfate salts are freely soluble. Particulate matter and dissolved organic matter may bind a substantial portion of cadmium in both freshwater and saltwater. Cadmium does not easily degrade in aquatic systems and tends to bind to sediments, but is also readily bio-accumulated by aquatic organisms. Bio-concentration factors (BCFs) in freshwater fish were as high as 12,400 and for a saltwater polychaete were as high as 3,160 (USEPA, 2001).
The BCFs for a saltwater fish were 48 from a 21-day exposure of the mummichog. However, among ten species of invertebrates, the BCFs range from 22 to 3,160 for whole body and from 5 to 2,040 for muscle. BCFs for five species of saltwater crustaceans range from 22 to 307 for whole body and from 5 to 25 for muscle, and whole-body BCFs for two species of grass shrimp, Palaemonetes pugio and P. vulgaris were reported as 203 and 307. The highest BCF was reported for the polychaete, Ophryotrocha diadema, which was attained a BCF of 3,160 after 64-days exposure using the renewal technique, although tissue residues had not reached steady-state (USEPA, 2001).
BCFs for four species of saltwater bivalve molluscs range from 113 for the blue mussel to 2,150 for the eastern oyster. In addition, the range of reported BCFs is rather large for some individual species. BCFs for the oyster include 149 and 677, as well as 1,220, 1,830 and 2,150. Similarly, two studies with the bay scallop resulted in BCFs of 168 and 2,040 and three studies with the blue mussel reported BCFs of 113, 306, and 710. The importance of metal speciation on cadmium accumulation in the soft tissues of Mytilus edulis was studied, where cadmium complexed as Cd-EDTA, Cd-alginate, Cd-humate, and Cd-pectate was bioconcentrated at twice the rate of inorganic cadmium. Because bivalve molluscs usually do not reach steady-state, comparisons between species may be difficult and the length of exposure may be the major determinant in the size of the BCF (USEPA, 2001).
Bioaccumulation is often a good integrative indicator of the organism exposures to metal in polluted ecosystems, but varies widely among metals, species, and environmental conditions, which could well be predicted by the dynamic multi-pathway bioaccumulation model (DYMBAM), capturing the biologically driven patterns that differentiate bioaccumulation among species and considering geochemical influences, biological differences, and differences among metals. It was noteworthy that the links between bioaccumulation and toxicity are complex, because bio-accumulated metal is not necessarily toxic. Toxicity is determined by the uptake of metal internally and the species-specific partitioning of accumulated metal between metabolically active and detoxified forms (Luoma and Rainbow, 2005).
Mechanisms of Cadmium Toxicity
The eco-toxicology of cadmium has put greater emphasis on gathering data on the sensitivity of species rather than understanding the reasons why one species is apparently more sensitive to cadmium than another, which drew the attention of researchers to the relationship between the potential toxicity of cadmium and to the risks presented by its accumulation in biota.
Metal Accumulation Patterns
Aquatic organisms can take up or accumulate trace metals in their tissues, via the surrounding aquatic environments or diet, based on different metal accumulation patterns, depending on whether these metals are essential to metabolism or not. The bio-accumulated concentrations are divided into two components—a metabolically available form and a stored detoxified form (Figure 9).
Figure 9. Generalized scheme showing the various compartments in which metals may be present and accumulate inside aquatic organisms (modified from Vijver et al., (2004)), [Ar] is metabolically metal pool, for nonessential metals this pool is negligible; [AE] is excess pool above metabolic requirements, causing toxicity and eventually mortality when elimination or detoxification fluxes are slower than uptake rate; [S] is storage; and [D] is detoxification.
Metals in the detoxified form can be excreted or bound to proteins or other particular molecules of high affinity. Cadmium exist in a variety of bound forms including being associated with metallothioneins (MTs) and/or granules (Rainbow, 2002).
Essential metals may be subject to regulation either by limiting metal uptake at the level of the total body concentration or by involving organism-specific accumulation patterns with active excretion from the metal excess pool and/or storage in an inert form and/or excretion of stored (detoxified) metal (Figure 9) (Vijver et al., 2005). For non-essential metals, excretion from the metal excess pool and internal storage without elimination are the major patterns of accumulation, and body concentrations generally increase with increasing external concentrations because there are no excretion and no essential requirements. On uptake, waterborne cadmium will be in a metabolically available forms and will need to be detoxified if the amount of metabolically available cadmium in the body is not to exceed the threshold for toxic effects. Most accumulated metal might be expected to be in detoxified form and toxic effects are instigated when [U] exceeds [D] (Figure 10) (Rainbow, 2002).
Figure 10. Cadmium accumulation pattern of aquatic organisms from waterborne sources showing net accumulation of cadmium without excretion of any cadmium taken up (modified from Rainbow, (2002)), [AE]—excess cadmium over and above metabolic requirement, [AT]—threshold concentration of metabolically available cadmium, above which the accumulated cadmium is toxic, [D]—metabolically available cadmium in excess of requirements is detoxified, [S]—the detoxified component of accumulated cadmium with no upper concentration limit.
Toxicity is related to a threshold concentration of metabolically available metal rather than to total accumulated metal concentration. The onset of toxic effects depends only on the concentration of accumulated metal in a metabolically available form. It follows, therefore, that toxicity occurs when the rate of metal uptake into the body exceeds the combined rate of excretion plus detoxification of metabolically available form. Organisms are able to control metal concentrations in certain tissues of their body to minimize damage of reactive forms of trace metals and to control selective utilization of essential metals (Vijver et al., 2005), but once the metabolically available forms passed a threshold concentration, the organism will then suffer toxic effects, initially sublethal but eventually lethal.



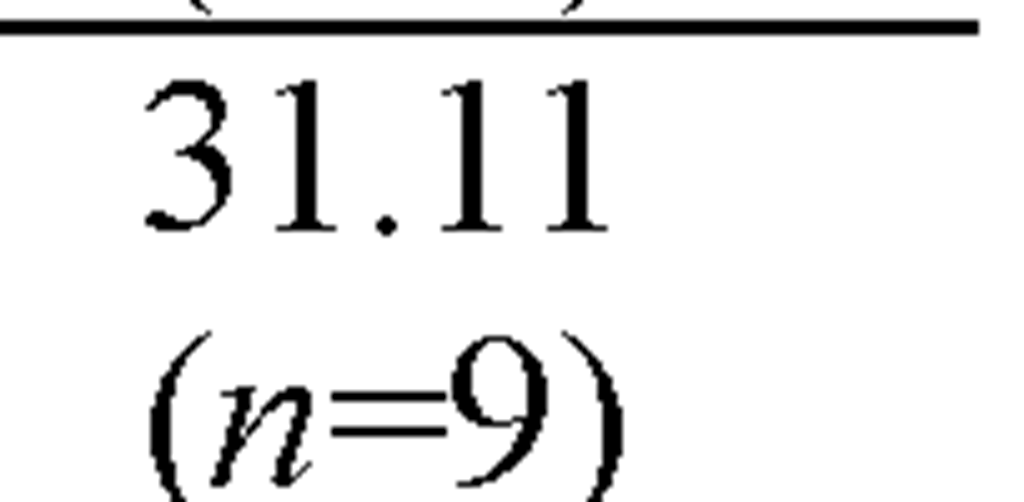
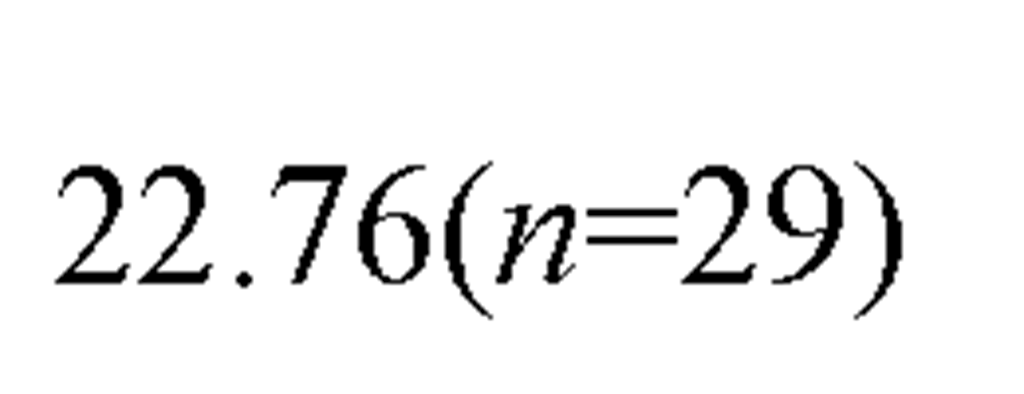

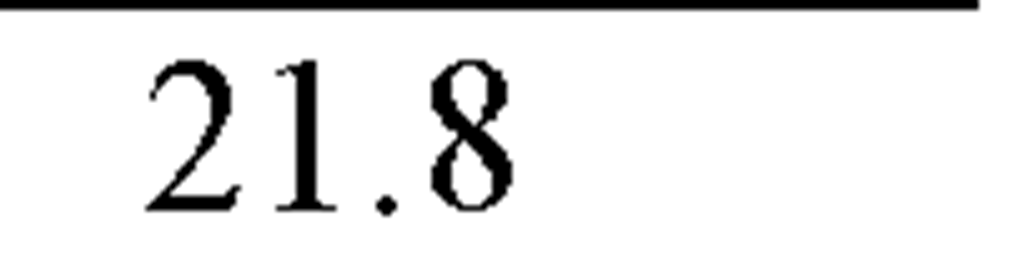
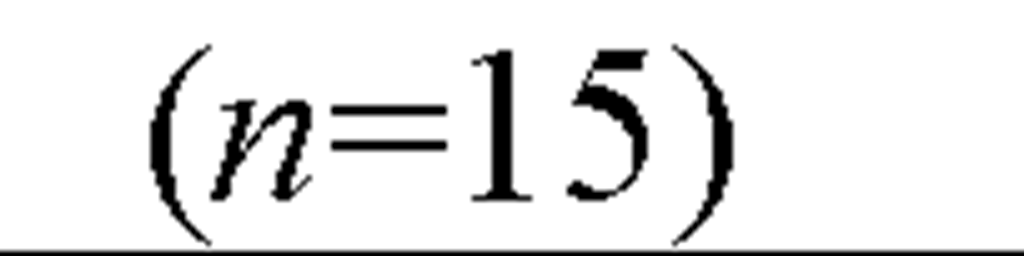
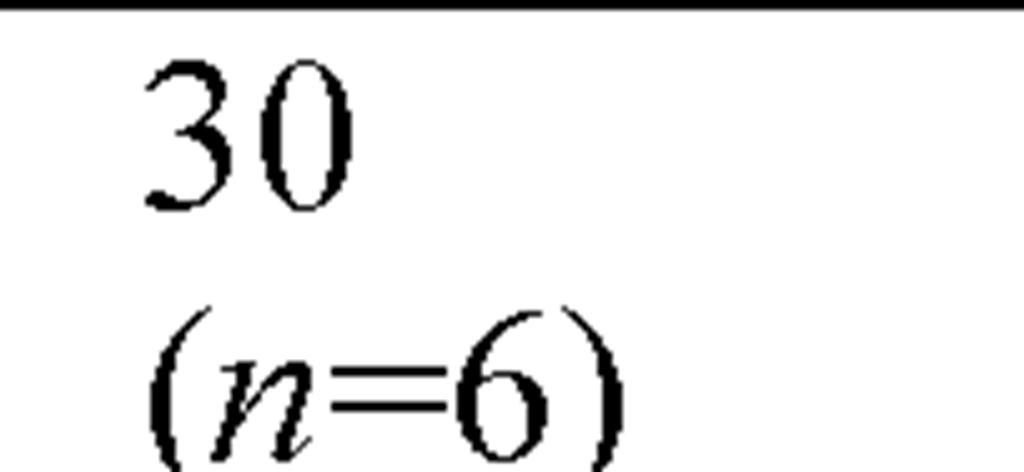

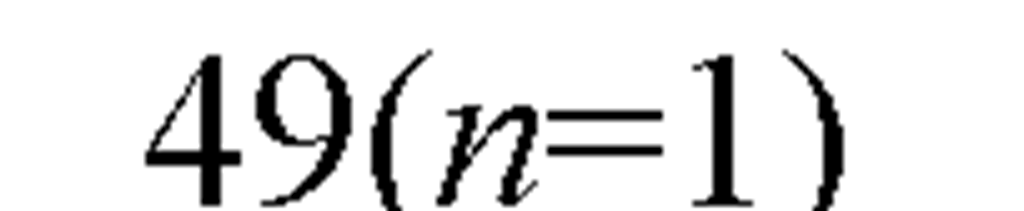

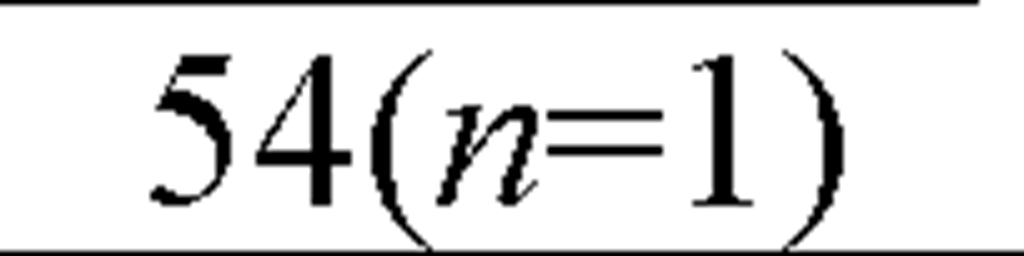
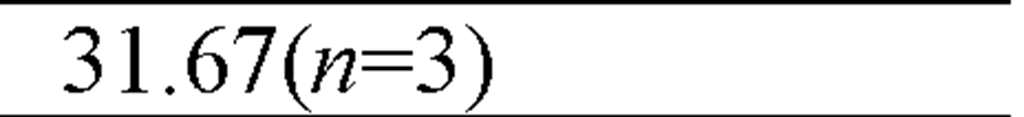


![Generalized scheme showing the various compartments in which metals may be present and accumulate inside aquatic organisms (modified from Vijver et al., (2004)), [Ar] is metabolically metal pool, for nonessential metals this pool is negligible; [AE] is excess pool above metabolic requirements, causing toxicity and eventually mortality when elimination or detoxification fluxes are slower than uptake rate; [S] is storage; and [D] is detoxification. Generalized scheme showing the various compartments in which metals may be present and accumulate inside aquatic organisms (modified from Vijver et al., (2004)), [Ar] is metabolically metal pool, for nonessential metals this pool is negligible; [AE] is excess pool above metabolic requirements, causing toxicity and eventually mortality when elimination or detoxification fluxes are slower than uptake rate; [S] is storage; and [D] is detoxification.](http://what-when-how.com/wp-content/uploads/2012/04/tmp4928_thumb.png)
![Cadmium accumulation pattern of aquatic organisms from waterborne sources showing net accumulation of cadmium without excretion of any cadmium taken up (modified from Rainbow, (2002)), [AE]—excess cadmium over and above metabolic requirement, [AT]—threshold concentration of metabolically available cadmium, above which the accumulated cadmium is toxic, [D]—metabolically available cadmium in excess of requirements is detoxified, [S]—the detoxified component of accumulated cadmium with no upper concentration limit. Cadmium accumulation pattern of aquatic organisms from waterborne sources showing net accumulation of cadmium without excretion of any cadmium taken up (modified from Rainbow, (2002)), [AE]—excess cadmium over and above metabolic requirement, [AT]—threshold concentration of metabolically available cadmium, above which the accumulated cadmium is toxic, [D]—metabolically available cadmium in excess of requirements is detoxified, [S]—the detoxified component of accumulated cadmium with no upper concentration limit.](http://what-when-how.com/wp-content/uploads/2012/04/tmp1429_thumb_thumb.png)
