Speciation and Bioavailability of Cadmium in Aquatic Environment
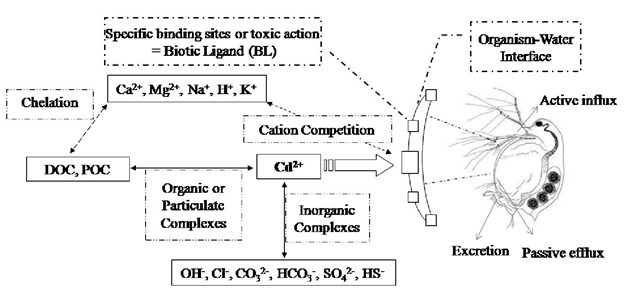
The free cadmium cation [Cd2+] has been shown to be the most bioavailable and toxic form of the metal for many aquatic organisms, which means that its bioavailability and toxicity is diminished when cadmium becomes associated with particulate matter or forms of inorganic or organic complexes. Bioaccumulation of cadmium from the aquatic environment is controlled by many interrelated aspects of water chemistry, which involve cadmium speciation in the water and the ways in which the cadmium species compete with chemical ligands in the water, with binding sites on both sedimentary and suspended particulate matter, as well as on organisms (Figure 5).
In freshwater, dissolved cadmium is present mainly in free ionic form, although ionic cadmium tends to associate with colloidal and particulate matter, and the carbonate and the hydroxides in soft waters of which become increasing important at higher pH. The extent to which these occur and the factors (particularly pH dependent) that influence the association of cadmium with particles has biological significance. Dissolved cadmium is directly available for uptake by biota, and accumulation or toxicity of cadmium has similarly been related to Cd2+ in freshwater studies, although many heterotrophs ingest particles and assimilate material from them, and the distribution coefficient that relates particulate to dissolved cadmium for a wide range of natural waters is relatively constant. In seawater, cadmium chemistry is dominated by chloride complexation, so 97% of total cadmium may be in the form of chloride complexes. CdCl+ and
A high proportion of cadmium is also associated with particles and is present as complexes in coastal and estuarine waters, (Mackay, 1983). Moreover, cadmium accumulation by marine animals and any resultant toxicity have often been shown to relate inversely to salinity (Wright and Welbourn, 1994).
Dissolved organic carbon (DOC) has the capacity to decrease the availability of cadmium by binding or complexing free cadmium, i.e., by changing cadmium speciation, bioavailability, and thus toxicity. Huebert and Shay (1992) indicated that cadmium bioavailability and toxicity in water may be greatly reduced by the addition of a strong complexing agent such as EDTA. Organic chelates such as humic acids are also likely to bind cadmium and appear antagonistic to the toxicity of cadmium. Although this binding is not as strong as EDTA, such studies of which implicated DOC in influencing cadmium bioavailability in the aquatic environment.
Figure 5. Conceptual diagram of cadmium speciation and cadmium-binding sites based on the biotic ligand model (BLM) showing inorganic and organic complexation in the water and interaction of metals and cations on the biotic ligand, adaptation to the zooplankton. (after Di Toro et al., 2001). POC=particulate organic carbon; DOC=dissolved organic carbon; CO32- =carbonate; HCO3-=bicarbonate; OH-=hydroxide; Cl-=chloride; SO42-=sulfate; HS-=sulfide.
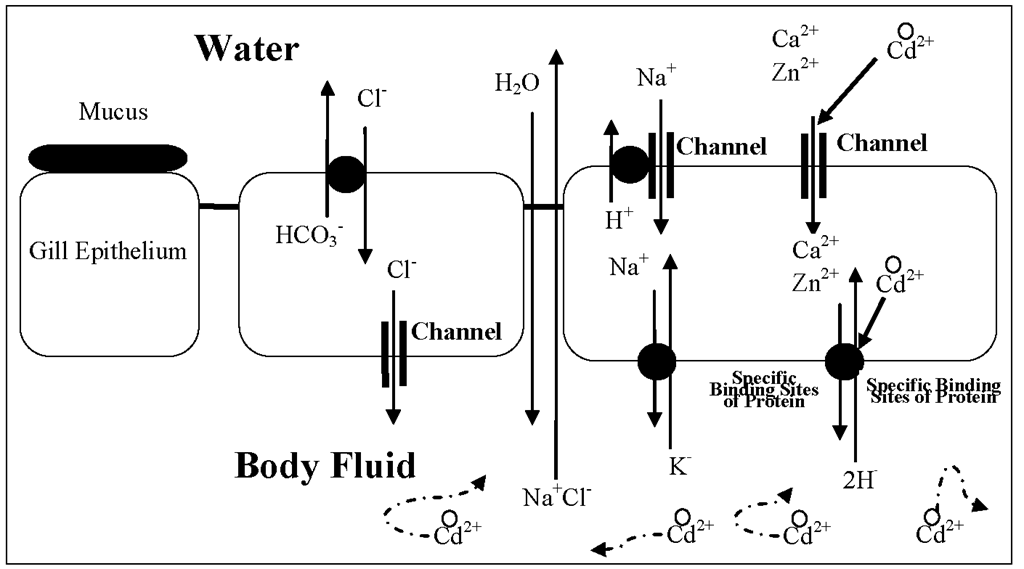
Many factors in aquatic environment might also affect the test results of the cadmium toxicity to aquatic organisms, for example, hardness (including calcium and manganese by influencing membrane integrity) and alkalinity are often thought of as having a major effect on the toxicity of cadmium in freshwater (Figure 6), although the observed effect may be due to one or more of a number of usually interrelated ions, such as hydroxide, carbonate, calcium, and magnesium.
In estuarine environments, where organisms are exposed to low and fluctuating salinities, cadmium speciation may be further complicated by other aspects of water chemistry, such as changes in organic carbon loading and significant alterations in the ratio of major to minor ions in the water body. In particular, the role of calcium in modifying cadmium availability may become increasingly important in fresher water.
In saline and also in freshwater environments, it is likely that the major effect of calcium on cadmium toxicity is through competition for organismal binding sites, rather than by altering its speciation. Calcium is antagonistic to cadmium uptake and release by the gill and may indirectly affect accumulation by other tissues.
The inverse relationship between calcium and cadmium is most easily explained as a competition for binding sites on the mussels (Figure 6).
Figure 6. Schematic diagrams of transport pathways of essential ions (Na+, Ca2+, Zn2+and Cl-) and their interactions with toxic cadmium cations (Cd2+) in the epithelium of aquatic organisms. After uptake (transport across the plasma membrane), free reactive Cd2+ will circulate through the body fluid and bind some proteins, then transport them into different compartments, where they are detoxified or not.
Furthermore metals such as zinc, iron and selenium can potentially influence the uptake and toxicity of cadmium by competing with cadmium for binding sites on epithelial and cell membranes of aquatic species or for other ligands that might affect cadmium availability indirectly, and hydrogen ion plays a role through the physiological processes and the cadmium speciation (Wright and Welbourn, 1994).
In the sedimentary environment, bioaccumulation of cadmium is largely dependent on soluble cadmium present in pore waters and may also depend upon the chemical forms of solid phases of cadmium, since the preponderance of available ligands, both organic and inorganic, has a major influence on cadmium availability (Nebeker et al. 1986). Despite the dominance of iron oxides in sequestering cadmium in aerobic sediments, humic acids have also been shown to possess significant cadmium binding capacity in freshwater sediments and may reduce the availability of cadmium to benthic organisms. Furthermore, cadmium is held partly in combination with carbonates and sulfides, and partly in complex organic combinations. In the latter example, cadmium combines with the sulfur-rich fractions of organic matter of which there is great excess, for the inorganic and organic contributions are widely variable. So the combined effects of pH, organic complexing agents, and redox potential of sediments need to be considered in assessing the availability of cadmium. The concentrations of acid-volatile sulfide have been advanced as a predictor of sediment cadmium toxicity based on the fact that any free Cd2+ in the pore waters will exchange with acid volatile sulfides (largely FeS) informing insoluble CdS (Di Toro et al., 1990).
The toxicity and impact of cadmium on aquatic organisms depends on different cadmium forms, which can have different toxicities and bio-concentration factors. In most well oxygenated freshwaters that are low in total organic carbon, [Cd2] will be the predominant form. Precipitation by carbonate or hydroxide and formation of soluble complexes by chloride, sulfate, carbonate, and hydroxide should usually be of little importance. In saltwater with salinities from about 10 to 35 %o, cadmium chloride complexes predominate. In both fresh and saltwater, particulate matter and dissolved organic material may bind a substantial portion of the cadmium, and under these conditions cadmium may not be bioavailable due to this binding. So the bioavailability of cadmium and, consequently, its accumulation in tissues depends on a number of factors.