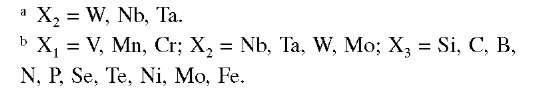True metals include the alkali and alkaline earth metals, beryllium, magnesium, copper, silver, gold, and the transition elements. These metals exhibit those characteristics generally associated with the metallic state.
The B subgroups comprise the remaining metallic elements. These elements exhibit complex structures and significant departures from typically metallic properties. Aluminum, although considered under the B subgroup metals, is somewhat anomalous in that it exhibits many characteristics of a true metal.
The alloys of a true metal and a B subgroup element are very complex, because their components differ electrochemically. This difference gives rise to a stronger tendency toward definite chemical combination than to solid solution. Discrete geometrically ordered structures usually result. Such alloys are also termed electron compounds. The aluminides are phases in such alloys or compounds. A substantial number of beta, gamma, and epsilon phases have been observed in electron compounds, but few have been isolated and evaluated.
The development of intermetallic alloys into useful and practical structural materials remains, despite recent successes, a major scientific and engineering challenge. As with many new and advanced materials, hope and the promise of major breakthroughs in the near future have kept a very active and resilient fraction of the metallurgical community focused on intermetallic alloys.
Compared to conventional aerospace materials, aluminides of titanium, nickel, iron, niobium, etc., with various compositions offer attractive properties for potential structural applications. The combination of good high-temperature strength and creep capability, improved high-temperature environmental resistance, and relatively low density makes this general class of materials good candidates to replace more conventional titanium alloys and, in some instances, nickel-base superalloys. Moreover, titanium aluminide matrix composites appear to have the potential to surpass the monolithic titanium aluminides in a number of important property areas, and fabrication into composite form may be a partial solution to some of the current shortcomings attributed to monolithic titanium aluminides.
The material classes include both monolithic and continuous fiber composite materials based on the intermetallic composition Ti3Al (a2-phase) and monolithic alloys based on the intermetallic composition TiAl (y-phase). In their monolithic form, and as a matrix material for continuous fiber composites, titanium alu-minides are important candidates to fill a need in the intermediate-temperature regime of 600 to 1000°C. Before these materials can become flightworthy, however, they must demonstrate reliable mechanical behavior over the range of anticipated service conditions.
The P and y phases that are found to exist in the Mo-Al alloy system are generally considered to correspond to the compositions MoAl5 and MoA12, respectively.
Powder metallurgy techniques have proved feasible for the production of alloys of molybdenum and aluminum, provided care is taken to employ raw materials of high purity (99% +). As the temperature of the compact is raised, a strong exothermic reaction occurs at about 640°C causing a rapid rise in temperature to above 960°C in a matter of seconds. Bloating occurs, transforming the compact into a porous mass. Complete alloying, however, is accomplished. This porous, friable mass can be subsequently finely comminuted, repressed, and sintered (or hot-pressed) to form a useful body quite uniform in composition. Vacuum sintering at 1300°C for 1 h at 0.04 |im produces clean, oxide-free metal throughout. Wet comminution prevents caking of the powder, and a pyrophoric powder can be produced by prolonged milling.
Hot pressing is a highly successful means of forming bodies of molybdenum and aluminum previously reacted as mentioned above. Graphite dies are employed to which resistance heating techniques are applicable. A parting compound is required since aluminum is highly reactive with carbon causing sticking to the die walls.
Hot-pressed small bars exhibit modulus of rupture strengths ranging from 40,000 to 50,000 psi at room temperature, decreasing to 38,000 to 40,000 psi at 1040°C. Room temperature resistance to fuming nitric acids is excellent.
As has been recognized for some time, ordered intermetallic compounds have a number of properties that make them intrinsically more appealing than other metallic systems for high-temperature use. The primary requirements for high-temperature structural intermetallics, as with any high-temperature structural material, are that they (1) have a high melting point, (2) possess some degree of resistance to environmental degradation, (3) maintain structural and chemical stability at high temperatures, and (4) retain high specific mechanical properties at elevated temperatures whether they are intended as monolithic components or as reinforcing fibers or matrix in composite structures.
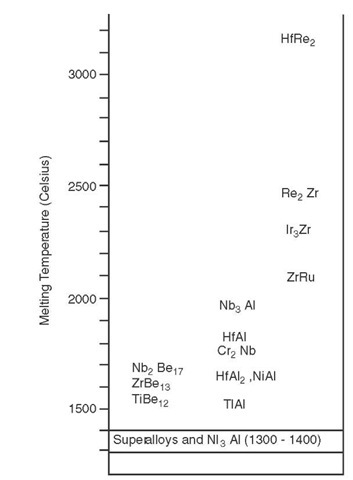
Melting point is a useful first approximation of the high-temperature performance of a material, as various high-temperature mechanical properties (e.g., strength and creep resistance) are limited by thermally assisted or diffusional processes and thus tend to scale with the melting point of the material. Therefore, the inter-metallics can be crudely ranked in terms of their melting points to indicate their future applicability as high-temperature structural materials.
As may be seen in Figure A.2, metallic materials (intermetallics or otherwise) that are currently in use or being studied melt at temperatures much lower than 1650°C. If these materials are discounted from consideration, the remaining intermetallics in Figure A.2 may be roughly divided into two groups: those that fall in the temperature range just above 1650°C and those whose melting points extend to much higher temperatures.
FIGURE A.2 Melting points of various intermetallic compounds relative to superalloys.
This second group of intermetallic compounds (IMCs) belongs to a group of interme-tallics that are predicted on the basis of the Engel-Brewer phase stability theory.
There are several techniques that have been developed and used to improve the toughness of intermetallics as well as intermetallic compounds:
• Crystal structure modification (macro-alloying)
• Microalloying
• Control of grain size or shape
• Reinforcement by ductile fibers or particles
• Control of substructure
Table A.7 includes the above major categories, however, the use of hydrostatic pressure and suppression of environment should also be cited.
Additions of chromium and manganese have induced appreciable compressive ductility and modest improvements in bend ductility of Al3Ti, but significant tensile ductility remains unattainable.
The fracture toughness of Ti3Al alloys also can be markedly improved by a control of composition, microstructure, and processing techniques. However, the maximum benefits are obtained at about 400°C.
Microstructural control has proved to be a particularly effective means of ductilizing TiAl and Ti3Al. It is now generally accepted that lamellar microstructures in TiAl, consisting of alternating y- and a2-plates, provide the highest ductility.
The interest in aluminides has covered the high-melting-point phases in metallic systems with aluminum.
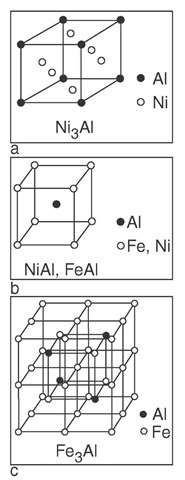
Ordered intermetallics constitute a unique class of metallic materials that form long-range-ordered crystal structures (Figure A.3) below a critical temperature that is generally referred to as the critical ordering temperature (Tc). These intermetallics usually exist in relatively narrow compositional ranges around simple stoichio-metric ratios.
The search for new high-temperature structural materials has stimulated much interest in ordered intermetallics. Recent interest has been focused on aluminides of nickel, iron, and titanium. These aluminides possess many attributes that make them attractive for high-temperature structural applications. They contain enough aluminum to form, in oxidizing environments, thin films of aluminide oxides that often are compact and protective. They have low densities, relatively high melting points, and good high-temperature strength properties. For example, Ni3Al shows an increase rather than a decrease in yield strength with increasing temperatures. The aluminides of interest are described in Table A.8.
Toughness and Ductility Improvements
B in Ni3Al, Ni3Si, PdIn
Be in Ni3Al
Fe, Mo, Ga in NiAl
Ag in Ni3Al
Macroalloying
Fe in Co3V
Mn, V, Cr in TiAl
Nb in Ti3Al
Mn, Cr in Al3Ti
Pd in Ni3Al
Grain Size Refinement
NiAl
Hydrostatic Pressure
Ni3Al
Martensite Transformation
Fe in NiAl
Composites (Fibers or Tubes)
NiAl/304SS
Al3Ta/Al2O3
MoSi2/Nb-1Zr
Composites (Ductile Particles)
Nb in TiAl
Fe, Mn in Ni3Al
Nb in MoSi2
In the range of 14 to 34% aluminum by weight occur the two intermetallic phases Ni3Al and NiAl. The alloys are prepared by powder metallurgy and by casting techniques. Compacts of NiAl + 5% Ni, produced by powder techniques, exhibit room-temperature modulus of rupture values of 144,000 psi, and heat shock resistance is considered excellent. Good resistance to red and white fuming nitric acids is obtained.
FIGURE A.3 The crystal structure of nickel and iron aluminides: (a) LI2, (b) B2, (c) DO3.
The cast alloys of nickel and aluminum exhibit an increasing exothermic character with increasing aluminum content. Alloys with the exception of the 34% aluminum alloy and NiAl (31.5% Al) produce sound castings free of excessive porosity. A room-temperature tensile strength of approximately 49,000 psi is exhibited by the Ni3Al compound with about 5% elongation. The 25% Al (NiAl) alloy has a room temperature tensile strength of 24,000 psi.
The 17.5% aluminum alloy that contained a mixture of the phases Ni3Al and NiAl exhibits the best strength properties. Room-temperature tensile strength is approximately 80,000 psi with about 2% elongation, while at 815°C the tensile strength is about 50,000 psi. This alloy can be rolled at 1315°C, and possesses good thermal shock and oxidation resistance. Impact resistance is fair. The 100-h stress-to-rupture strength at 734°C is 14,000 psi, but, it should be noted, creep rates are high.
Ni3Al is an intermetallic phase that forms at the nickel-rich end of the Ni-Al system and has a crystal structure (Figure A.3). Ni3Al is the most important strengthening constituent of nickel-base superalloys. This is because the alu-minide possesses an excellent elevated-temperature strength in addition to good oxidation resistance. Unlike conventional materials, Ni3Al and its alloys show a yield anomaly; that is, its yield strength increases rather than decreases with increasing temperature.
Boron has been found to be most effective in improving the tensile ductility of Ni3Al (<25% aluminum) tested in air at room temperature. Other tests indicate that boron-doped Ni3Al is not sensitive to test environment at room temperature.
Boron-free Ni3Al (24% Al) showed a ductility of only 8.2% in dry O2 while boron-doped Ni3Al exhibited 42.8%. This suggests that boron segregation also enhances the grain-boundary cohesion in Ni3Al. In addition to boron, iron, chromium, and zirconium appear to enhance the grain-boundary properties and improve the tensile ductility of Ni3Al at room temperature.
The mechanical properties of Ni3Al strongly depend on deviation from alloy stoi-chiometry (i.e., aluminum/nickel ratio) and ternary alloying additions. The aluminide is capable of dissolving substantial alloying additions without forming second phases; as a result, its deformation and fracture behavior can be strongly altered by alloying.
Mechanical and metallurgical properties of Ni3Al can be improved by alloying additions. Recent alloy design efforts have led to the development of Ni3Al-base alloys with the following composition range (in atomic percent) for structural use at elevated temperatures in hostile environments: Ni-(14 to 18%) Al-(6 to 9%) Cr-(1 to 4%) Mo-(0.01 to 1.5%) Zr/Hf-(0.01 to 0.20%) boron.
NiAl containing more than about 41 at% forms a single-phase ordered B2 structure based on the body centered cubic (bcc) lattice (Figure A.3). In terms of thermophysical properties, B2 NiAl offers more potential for high-temperature structural applications than Ll NiAl. It has a higher melting point (1638°C), a substantially lower density (5.86 g/cm2), and a distinctly higher thermal conductivity, 76 W/(m x K), at ambient temperatures. In addition, NiAl has excellent oxidation resistance at high temperatures.
The structural use of NiAl suffers from two major drawbacks: poor fracture resistance at ambient temperatures and low strength and creep resistance at elevated temperatures. Single crystals of NiAl are quite ductile in compression, but both single-crystal and polycrys-talline NiAl appear to be brittle in tension at ambient temperatures.
Properties of Nickel and Iron Aluminides
|
Critical Ordering Temp. |
Melting Point |
Material Density |
Young’s Modulus |
||
|
Alloy |
Crystal Structure3 |
(°C) |
(°C) |
(g/cm3) |
(Gpa) |
|
Ni3Al |
L12 (ordered fcc) |
1390 |
1390 |
7.50 |
178 |
|
NiAl |
B2 (ordered bcc) |
1640 |
1640 |
5.86 |
294 |
|
Fe3Al |
D03 (ordered bcc) |
540 |
1540 |
6.72 |
141 |
|
B2 (ordered bcc) |
760 |
1540 |
|||
|
FeAl |
B2 (ordered bcc) |
1250 |
1250 |
5.56 |
260 |
a fcc = face-centered cubic; bcc = body-centered cubic.
Because of the potential use of NiAl at high temperatures, considerable effort has been devoted to understanding brittle fracture and improving mechanical properties of NiAl during the past years.
The room-temperature tensile ductility of NiAl is increased from 1% to as high as 6% by adding about 0.2 at% iron. The ductility decreases sharply when the iron content is beyond 0.4 at%. A similar effect has been observed for molybdenum and gallium.
In addition to its widespread popularity as a model system for the basic understanding of a broad range of physical and mechanical properties, NiAl is a material with several potential engineering applications. Coatings currently comprise the principal engineering use of NiAl and NiAl-base alloys, and several other potential applications are in different stages of research and development.
Applications Coatings
NiAl is the basis of a family of oxidation- and corrosion-resistant coatings that have been used on nickel-base and cobalt-base superalloys over the past 30 years. NiAl coatings 25 to 100 mm thick are typically applied by a pack cementation process.
Both inward diffusion and outward diffusion coatings have been applied. The mechanisms of coating degradation include a depletion due to spallation and reaction with the substrate. A variety of alloying additions, most notably chromium and yttrium, have been applied to improve performance in thermal cycling and hot corrosion.
Turbine Blades and Vanes
The advantages associated with NiAl as a candidate structural material in advanced gas turbine engines include a 30% reduction in density over current nickel-base superalloys, good intrinsic environmental resistance, a thermal conductivity three to eight times larger than nickel-base superalloys (for improved cooling efficiency), and a melting temperature that is approximately 128°C higher than superalloys (for higher operating temperatures).
The processing, fabrication, and engineering design of NiAl, along with an understanding and improvement in impact properties, will also provide significant technical challenges that must be overcome before NiAl can be successfully employed as a structural material that may compete with current nickel-base superalloys. It is important to point out that a single crystal approach is crucial to obtaining a balance of both room-temperature ductility and high-temperature strength. Although NiAl-base composites may provide an alternative avenue to balanced properties, this approach is still in its infancy.
Electronic Applications
NiAl is an attractive material for metallizations on III-V semiconductors, and is also a critical component in complex "metal"/ III-V semiconductor heterostructures, such as enhanced-barrier Schottky contacts and semiconductor-clad metallic quantum wells. The characteristics that make NiAl an attractive candidate include a good lattice parameter match with several semiconductors (for epitaxy), chemical stability, good electrical conductivity, and a stable native oxide that allows patterning in air without destroying electrical continuity. An NiAl layer 3.3 mm thick is currently the thinnest independently contacted electrode in any solid-state device.
Among the alloys currently being considered as replacements for superalloys in high-temperature applications, some of the most promising are the intermetallic compounds in the Nb-Al system. The three compounds NbAl3, Nb2Al and Nb3Al have melting temperatures in excess of many candidate replacement materials and densities that are superior to or competitive with those materials. Furthermore, the crystal structures, although complex, are reasonably well understood. In addition to these physical properties, there is a distinct processing consideration that leads to heightened interest in this system. The compounds of the Nb-Al system represent the highest melting temperature alloys that can be processed using currently active superalloy techniques, i.e., the various vacuum and hearth melting techniques.
The intermetallic compounds of the Nb-Al system, when systematically viewed as a whole, demonstrate both an orderly progression of high-temperature properties and a great deal of promise for future applications. Vickers micro-hardness as a function of temperature proceeds from NbAl3, which is inferior to Ni3Al, to Nb3Al, which is the highest-melting-temperature compound with a temperature superiority of about 600°C over Ni3Al.
Iron aluminides based on Fe3Al and FeAl (Figure A.3) have excellent oxidation and corrosion resistance because they are capable of forming protective oxide scales at elevated temperatures in hostile environments. These alu-minides exhibit corrosion rates lower than those of promising iron-based alloys (including coating material) by a couple orders of magnitude when tested in a severe sulfidizing environment at 800°C. The major drawbacks of the alu-minides are their poor ductility and brittle fracture at ambient temperatures and poor strength and creep resistance above 600°C.
Iron aluminides have been known to be brittle at room temperature for more than 50 years; however, the major cause of their low ductility and brittle fracture was not identified until recently. The study of the environmental effect on tensile properties indicates that the poor ductility commonly observed in air tests is caused mainly by an extrinsic effect — environmental embrittlement.
The understanding of the cause of brittle-ness in iron aluminides has led to new directions in the design of ductile iron aluminide alloys. The schemes used to improve the ductility of Fe3Al-FeAl alloys include control of surface conditions, reduction of hydrogen solubility and diffusion by alloy additions, refinement of grain structure by thermomechanical treatment, enhancement of grain-boundary cohesion by microalloying, and control of grain shape and recrystallization condition. The ductile Fe3Al alloys show a distinctly high yield strength and a high ductility of 13 to 16% when tested in air at room temperature. Similarly,FeAl (35.8 at% Al) alloys show an increase in room-temperature ductility from 2 to 11% as a result of alloying additions (zirconium, molybdenum, and boron) and grain-structure refinement. This ductility further increased to 14% by formation of protective oxide scales on specimen surfaces through preoxidation at 700°C. The room-temperature ductility of Fe3Al and FeAl can be significantly improved by refining grain structure via powder extrusion with or without second-phase particles (such as TiB2). The strength of Fe3Al and FeAl alloys at elevated temperatures can be improved by alloying with molybdenum, zirconium, niobium, titanium, and TiB2.
Gamma alloys of titanium aluminide can be grouped into single-phase (y)-alloys and two-phase (a2 + Y)-alloys (Table A.9). Single-phase alloys attracted attention initially because of their excellent resistance to environmental attack (e.g., oxidation and hydrogen absorption). In spite of a lack of progress toward overcoming their poor ductility and fracture toughness, however, interest in these engineering alloys has not diminished. Gamma titanium alu-minide alloys of importance are thus two-phase alloys based on Ti -(45 to 49%) Al with appropriate combinations of alloying elements from these groups designated X1, X2, and X3. (The footnotes in Table A.9 list the symbols of elements comprising the three groups.) The group X1 elements increase ductility in two-phase alloys. For example, an addition of 1 to 3 at% V, Cr, Cr + Mn, or V + Cr to Ti-48Al nearly doubles the room-temperature ductility of the material.
Compositions of Multicomponent Gamma Titanium Aluminide Alloys
|
Compositions (at%) |
|
|
Single phasea |
Ti-(50-52)Al-(1-2)X2 |
|
Two phaseb |
Ti-(44-49)Al-(113)X1- |
|
(1-4)X2-(0.1-1)X3 |
Additions from either group X1 or X2 elements strengthen the alloys by solid solution strengthening, with chromium appearing to be the most effective and manganese the least.
The group X1 elements are known to reduce oxidation resistance of gamma alloys. The X2 elements do not increase the ductility of the Ti-48Al-base alloys; however, they are very effective in improving oxidation resistance. Such improvements in both oxidation resistance and solid solution strengthening are two reasons almost all multicomponent alloys developed to date contain at least 2 at% niobium. Small additions of the group X3 elements have various effects wherein carbon and N2 are known to improve creep resistance, silicon, boron, nickel, and iron decrease the melt viscosity, and silicon may yield some improvements in oxidation resistance and room-temperature ductility. Minor additions of phosphorus, selenium, or terbium have recently been shown to result in remarkedly increased oxidation resistance.
Processing of Gamma Alloys
Gamma alloys may be processed by:
• Casting
• Ingot metallurgy (IM)
• Powder metallurgy (P/M)
• Sheet-forming
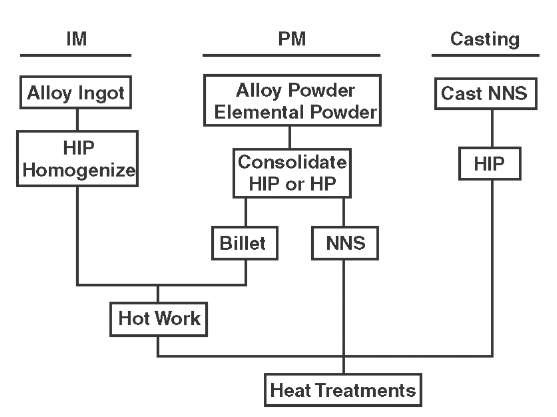
The XDTM process for producing gamma alloys containing TiB2p can be classified separately, although the XD alloys are produced by either the casting process or the ingot metallurgy process. Each process can involve one or more routes. Figure A.4 is a flowchart for typical processing methods and their specific routes.
The conventional method of producing foils from brittle materials was chemical milling. This approach involves suspending hot-rolled and surface-ground 0.050-mm-thick plates of the material in a pickling bath, and dissolving the metal until the plates are about 0.015 mm thick. For y-TiAl, the chemical milling technique yields a poor surface finish on the foil.
FIGURE A.4 Typical processing routes for gamma titanium aluminite alloys. HIP = hot isostatic pressing, HP = hot pressing, and NNS = near-net-shape.
Engineers knew that the y-TiAl, while brittle in tension, accepts high compression strains without failing. So they designed a new "iso-baric" rolling mill to maintain triaxial compression on the material as it passes through the rolls. This proprietary process increases yield from the original ingot to around 50%, and provides thinner (0.03 to 0.05 mm) foils with smaller grains. As a result, isobaric cold rolling is a breakthrough technology. A cold-rolled and annealed y-Ti-48-2-2 strip has a hardness of 260 Hv (Vickers).
Other processing methods include the self-propagating high-temperature synthesis (SHS) process, which starts with a structure of alternating layers of pure metal sheets or foils (e.g., iron, nickel, or titanium) and aluminum sheets or foils. The ductile metal layers are formed into the desired shape, either before or during the SHS reaction. The SHS reaction is initiated by heating in a vacuum. When the system reaches the initiation temperature (typically less than 660°C, the melting point of aluminum), an SHS reaction begins at the metal interface, i.e., between the aluminum and the iron, nickel, or titanium metals. The heat generated from the reaction melts the aluminum allowing rapid reaction with adjacent metal layers. That is, the SHS reaction occurs between the elemental metals producing well-bonded layers of unreacted elemental metal and metal-aluminum intermetal-lics. In this technique, the aluminum completely reacts, forming a titanium-aluminide-titanium,a nickel-aluminide-nickel, or an iron-alu-minide-iron layered composite structure.
A second technique takes elemental powder mixtures that are placed between layers of an elemental metal foil or sheet, after which the entire layup is heated under pressure in a vacuum. Starting powders have been iron, nickel, or titanium mixed with aluminum. Starting elemental metal sheets have been titanium, nickel, or iron.
Combining p/m (Extrusion), Rolling, and spf (Superplastic Forming)
A mixture of titanium and 34 wt% aluminum, i.e., 48 at%, was prepared by blending commercially available elemental powders with a maximum grain size of 100 mm compacted at room temperature in a uniaxial pressing machine to billets of 50 mm in diameter.
Titanium aluminide foils were produced using a combination of a standard P/M technique (extrusion), aluminum technology (rolling), and advanced titanium technology (SPF device). Claims have been made that since these techniques were available on a large scale, it should be possible to produce titanium alu-minide foils in production.
A series of low-pressure turbine blades fabricated from titanium aluminide was successfully tested and this development could eventually result in reducing the weight of aviation gas turbines by hundreds of pounds. 49% Ti-47% Al-2% Cr-2% Nb blades, which have about half the weight of comparable components made from conventional nickel-base alloys, were run in the fifth low-pressure turbine stage of a CF6-80C2 power plant and during the tests the blades went through 1000 flight cycles. Compared with traditional nickel-base alloys, the material has half the density and is comparable in strength up to about 760°C. The titanium aluminide also is about 50% stiffer than conventional titanium alloys. If used in the low-pressure section of new aircraft engines as blade material, the titanium aluminide could cut engine weight by more than 136.01 kg.
The 98 blades used in the tests weighed 217 g. Comparable nickel alloy components weigh 383 g. In an ideal situation, the lighter blades would also allow turbine wheels to be lighter and less robust because the reduced-weight blades create lower stresses during operation. The titanium aluminide alloy used to fabricate the blades were solid airfoils and were cast using conventional foundry techniques.
Future IM Developments
New titanium aluminide alloys, stronger and tougher than conventional a2(Ti3Al) materials, are based on the ordered orthorhombic-phase Ti3AlNb (Ti-25 at% Al-25 at% Nb).
The materials are potential weight-saving alternatives to nickel-base superalloys in relatively low temperature (650°C) aircraft gas-turbine engine applications. Several examples include exhaust-nozzle structures, compressor casings, and various compressor components; while the density of Ti3AlNb-base alloys (and other titanium aluminides) is less than two thirds that of nickel-base superalloys, such as Alloy 718, the lower weight can be translated into higher thrust-to-weight ratios or gains in fuel efficiency.
Of two new alloys, the Ti-22% Al-27% Nb has the best combination of high-temperature strength and room-temperature ductility and fracture toughness. The alloy is much stronger than conventional a2 titanium aluminides such as Ti-24% Al-11% Nb and Ti-25% Al-10% Nb-3% V-1 % Mo. It also has a higher strength-to-weight ratio, even though it is slightly denser. For example, Ti-22% Al-27% Nb has a factor-of-two advantage in strength-to-weight ratio over Ti-25% Al-10% Nb-3% V-1% Mo, an a2-alloy, from room temperature to 650°C. Fracture toughness (Klc) of Ti-22% Al-27% Nb averages about 28 MPa m1/2, which is higher than those of the a2-alloys Ti-25% Al-10% Nb-3% V-1% Mo (Kc = 14 MPa m1/2) and Ti-24% Al-17% Nb-1% Mo (19 MPa m1/2). Creep behavior of the Ti3AlNb-base alloy is competitive with lower toughness titanium aluminides that have been optimized for creep resistance.