Low back pain from facet joint arthritis or degeneration (anatomy and biomechanics, clinical presentation and physical examination, diagnostic imaging)
Anatomy and pathogenesis of facet joints pain
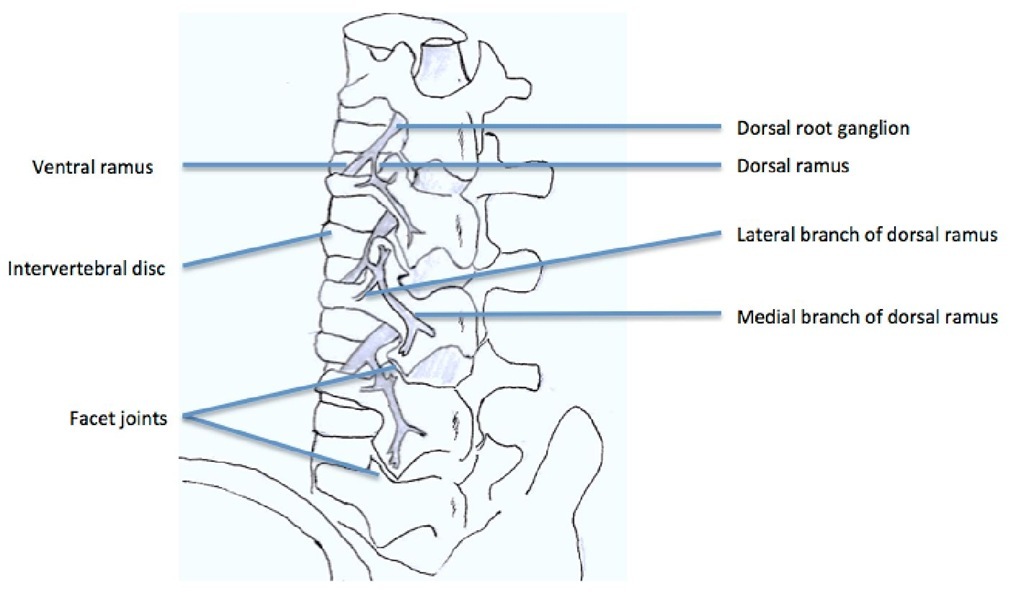
Each vertebra has two sets of facet joints. One pair faces upward (superior articular facet) and one downward (inferior articular facet). The joint surfaces are coated with cartilage allowing joints to move smoothly against each other. These joints allow flexion (bend forward), extension (bend backward), and twisting motion. Since these joints are just like other joints in the whole body, and they share axial loading with IVD, they can be affected by degeneration and arthritis. Facet joints have been implicated as a cause of chronic spinal pain in 15% to 40% of patients with chronic low back pain (Schwarzer et al., 1995a; 1995b). They are innervated by medial branches of dorsal rami from the spinal nerves (Fig. 3) and theoretically, facet joint pain can be treated by denervation of the medial branches of the dorsal rami, which supply the sensory innervation of the joints (Shealy, 1976; Bogduk & Long, 1980; Dreyfuss et al., 2000).
The lumbar facet syndrome was first described by Ghormley in 1933 (Ghormley, 1933). After detailed anatomical study of the lumbar zygapophysial nerve supply by Bogduk and Long in 1979 (Bogduk & Long 1979), several control studies showed initial benefits for pain relief by radiofrequency medial branch neurotomy (Dreyfuss et al., 2000; van Kleef et al., 1999). There are several diseases that contribute to facet joint disease, including degeneration, synovial cysts, ankylosing spondylitis, and trauma. A controlled trial has shown that RF medial branch neurectomy is not a placebo (van Kleef et al., 1999) and an observational study has shown that, provided patients are carefully selected using controlled diagnostic blocks, and provided a correct surgical technique is used, some 60% of patients can expect at least 80% relief of their pain at 12 months, and 80%of patients can expect at least 60% relief (Dreyfuss et al., 2000).
The nerve supply of the facet joint originates from two levels (Fig. 3); one branch of the primary ramus arises from the nerve root at the same level as the joint and another branch from the level above. Therefore therapeutic injection of the facet joint should include the joint above the suspected level (Lynch & Taylor 1986). In the lumbar region, the medial branch of the posterior ramus lies in a groove on the base of the superior articular facet, where it lies in direct contact with the base of the superior surface of the transverse process, passing between the mammillary and accessory processes. The nerve actually passes under the mammilloaccessory ligament, and this is the most reliable site for locating the nerve in the lumbar spine. Studies support the idea that RF denervation is a treatment of choice for initial pain relief; however, it does not produce permanent pain relief because the nerve eventually regenerates, usually within 12-18 months (North et al., 1994). Lord et al. found the median time of return to 50% of pre-procedure pain was 263 days (Lord et al., 1996). Dreyfuss et al. found that pain relief may last for about 12 months (Dreyfuss et al., 2000). So, repeated treatment may be necessary in some patients.
Fig. 3. The branches of lumbar roots
Clinical presentation and physical examination of lumbar facet syndrome
Like other types of LBP, pain from facet joint arthritis or degeneration is always related to other pathologies. So there is no specific clinical presentation or physical examination finding for lumbar facet syndrome. Because the lumbar facet joints are like other synovial joints, the pain related to arthritis causes local tenderness of affected joints. All of the lumbar facet joints are capable of producing some referred pain. Pain emanating from the upper facet joints tends to extend into the flank, hip, and upper lateral thigh, whereas pain from the lower facet joints is likely to penetrate deeper into the thigh, usually laterally and/or posteriorly. Infrequently, the L4-L5 and L5-S1 facet joints can provoke pain extending into the lower lateral leg and, in rare instances, even the foot (Cohen & Raja 2007).
Image diagnosis and diagnostic block
Lumbar facets hypertrophy, joint interface widening, adjacent neuroforamen and spinal canal narrowing are easily seen in CT and MRI studies. However, the clinical symptoms and image findings often show no correlation (van Kleef et al., 2010). Diagnostic blocks using local anesthetics are performed either in the joint space or medial branch nerve region. However, both are associated with significant false-positive and false-negative rates (Cohen & Raja 2007). Technically, half way between the upper edge of the transverse process and the ligamentum mammilloaccessorium was suggested by Dreyfuss et al. because the infiltration of the anesthetic agent will influence the proximal segmental nerves and caused false-positive results (Dreyfuss et al., 1997). Because the double block causes a high false-negative rate, it was not recommended for use right now (Bogduk & Holmes 2000).
Sacroiliac joint pain (anatomy and biomechanics, clinical presentation and physical examination, diagnostic imaging, intraarticular diagnostic block)
Sacroiliac joint (SIJ) pain was believed one of the causes of chronic LBP and single anesthetic and steroid injection has proved 35% effectiveness in patients who underwent failed fusion surgery (van Kleef et al., 2010). The prevalence of SIJ pain is from 16-44% depending on different diagnostic tools (Cohen 2005). Since this kind of pathology can be treated as a single block or minimally invasive procedure, data for SIJ pain have accumulated in recent years. RF applying to SIJ is to block the nerve fibers to the SIJ. The treatment efficacy, possible mechanisms and techniques will be discussed later.
Anatomy and biomechanics
The SIJ is one of the origins of chronic low back pain that has always been neglected (Maigne & Planchon 2005). It is the largest axial joint in the body, with an average surface area of 17.5 cm2 (Cohen 2005). The anterior third of the intersurface between the sacrum and the ilium is the true synovial joint and the rest of the junction is comprised of a strong and complicated ligamentous network. The ligamentous network of women is weaker which allows motility for parturition. Like other true synovial joints, age-related change, degeneration or arthritis developed on the cartilage surface of the SIJ. Each SIJ is composed of the true synovial joint and part of the ligamentous network.
The pain sources from SIJ can be divided into two parts, one is intra-articular and the other is extra-articular. The intra-articular SIJ pain may be due to the degenerative process of the articular cartilage and chronic arthritis, or autoimmune arthritis. Cohen reviewed the SIJ pathology and summarized some risk factors, including leg length discrepancy, gait abnormality, prolonged vigorous exercise, scoliosis, and spinal fusion to sacrum. (Cohen 2005) The other pain origin of SIJ is extra-articular structures such as ligaments weakening and muscle trauma and inflammation and hypermobility of SIJ caused by iatrogenic trauma. The innervation of the SIJ is divided into two parts, anterior and posterior. The posterior nerve supply to the SIJ comes from the lateral branches of the L4-S3 dorsal rami and the anterior innervation of SIJ is from the ventral rami of L4-S2 (Cohen 2005).
Clinical presentation, physical examination and diagnostic imaging
Since SIJ pain could be caused by either intra-articular or extra-articular structures, the diagnosis of SIJ pain is more complicated. Except for the risk factors in the medical history, physical examinations which involve the distraction of these joints can be helpful; for example, Patrick’s test and Gaenslen’s test. However, inconsistent predictable values were found in both physical examinations and medical history. Radiological studies showed the diagnosis of SIJ pathology is not correlated to the clinical presentation.
Diagnostic block of sacroiliac joint pain
Intraarticular anesthetics injection (diagnostic block) of SIJ can reasonably relieve the pain from SIJs. However, there are limited data supporting the diagnostic tool that strongly reflects the real pathology. Besides, the SIJ is technically difficult to approach even by a good experienced pain physician. The local anesthetics leakage to surrounding structures may cause false-positive results. Even with the CT-guided injection method, there is less than a 30% accomplishment rate for adequate injection (Rosenberg et al., 2000). According to these findings, diagnostic SIJ block is not reliable to diagnose true SIJ pain.
Low back pain combined with radicular pain (anatomy and biomechanics, clinical presentation and physical examination, diagnostic imaging, epidural nerve block)
Before the development of PRF (non-thermal), RF has been used directly targeting neural tissue, including movement disorders (Carr 1971; Cala et al., 1976), cardiac arrhythmias (Baszko et al., 2002), trigeminal neuralgia, and cervical and lumbar radicular pain, and the treatment target is the reflecting DRG (van Kleef et al., 1996). After PRF was developed, it was used more and more because of a lower complication rate (Chua et al., 2010). Although lacking good randomized control studies, RF or PRF applying to adjacent DRG of low back or radicular pain can still be used for selected patients before moving to surgical treatment.
Anatomy and biomechanics
The mechanisms of lumbosacral radicular (LSR) pain can be mainly divided into two possibilities. The first one is mechanical compromise and the other is chemical irritation of adjacent nerve roots. It is difficult to make a clear distinction between them. The mechanical compromise of nerve roots can cause local inflammatory cytokines up-regulation and the chemical irritation of nerve structure can cause nerve swelling and further compromise (Yang et al., 2011). Sometimes, either decompression or epidural steroid injection alone fails to relieve the clinical symptoms of LBP and LSR pain. Blocking or modulation of nerve transmission after inflammation and mechanical vicious circle calm down seems like an alternative treatment.
Clinical presentation and physical examination
LBP with LSR pain are common combined situations in clinical practice. There are two reasons which could explain this. First, the lumbosacral plexus is complicated and the branches from the surrounding structure of the spinal column often cause multiple segments pathology of the lower lumbar and sacral spine. Second, the mechanical compression of the spinal canal also frequently compromises the adjacent DRG or nerve roots which cause the dual symptoms. The clinical presentation of these patients is not easy to differentiate from other pathologies. Generally speaking, compromise of the neuronal structure causes neurologic dermatomes sensory impairment, motor weakness and neurogenic claudication. Physical examinations should be carefully taken, including neurologic signs.
Epidural steroid injection and diagnostic imaging
X-ray studies should be taken in chronic LBP and LSR pain patients including standard anteroposterior and lateral aspects. Dynamic flexion extension lateral film is required to differentiate the possibilities of spondylolisthesis, congenital lumbar stenosis, scoliosis and some unusual osteolytic lesions. A three dimensional view can be seen in CT and MRI studies. CT has benefits of more clear bony structures, including neuroforamens. MRI provides clear information of the spinal canal, nerve roots and disc herniation as well as unusual neogrowth or infectious diseases.
Selective adjacent epidural steroid injection calms down the inflammation process and nerve swelling in both mechanical and chemical irritation. Benny reviewed the efficacy of epidural injection and concluded that there was strong evidence for transforaminal injections in the treatment of LSR pain for both short term and long term relief (Benny & Azari 2011). Once the epidural injection works for pain relief of these patients, repeated injection was suggested if it recurs. Injection at least three times was suggested before shifting to more invasive procedures. RF or PRF for selective DRG treatment should be considered if the result of epidural injection is only temporary.
Procedures
Radiofrequency for intradiscal thermotherapy (evidence of hypothesis, indication, effect of procedure)
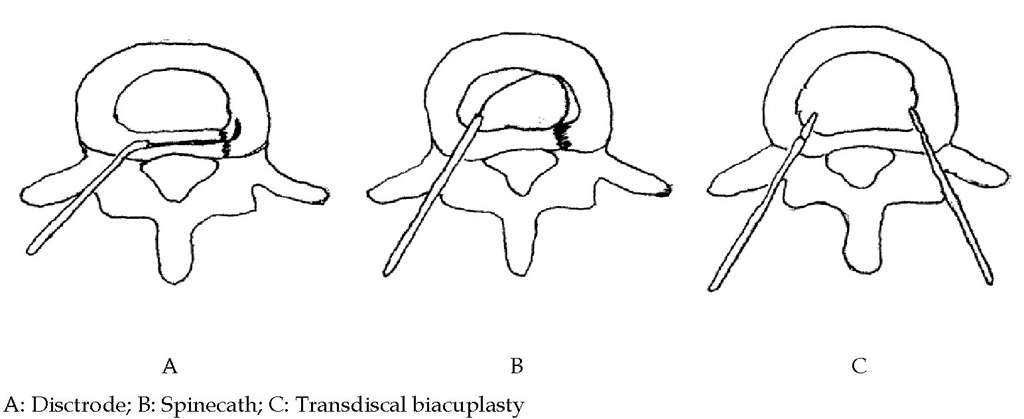
The intradiscal electrothermal therapy (IDET) is based on the mechanisms of AF tear healing and could be enhanced by thermal effect. The intradiscal volume and pressure decrease as well. Biologic study showed there are several types of radiofrequency intradiscal thermoplasty including spinecath (Oratec Interventions, Inc., Menlo Park, CA), disctrode, transdiscal biacuplasty (Fig. 4). The IDET procedures of spinecath and disctrode use a navigable intradiscal catheter with a thermal resistive coil. The procedure was performed under local anesthesia with lidocaine. All catheter placements are under fluoroscopy guidance. Preoperative administration of intravenous antibiotics two hours before the procedure is suggested.
In spinecath, the operator uses a 30-cm catheter with a 5-cm active electrothermal tip inserted anteriorly into the annulus or nucleus via a 17-gauge introducer. The active tip was advanced anterior-laterally inside the nuclear tissue and directed circuitously to return posteriorly, providing an ideal position to heat the entire posterior annulus. Once a satisfactory position was obtained in the anteroposterior, lateral views, the catheter was connected to a lead and passed to an independent technician. In all cases, the catheter tips were within 5 mm of the posterior vertebral margin upon review of saved fluoroscopic films. The disctrode was designed with a different approach which let the catheter directly pass through the posterior part of AF. The temperature during IDET begins at 65°C and was increased incrementally by 1°C every 30 seconds to achieve a final temperature of 90°C. The final temperature was maintained for 4 minutes, giving a total treatment time of 16.5 minutes.
The procedures of transdiscal biacuplasty include two RF electrodes. The symptomatic disc was reached in oblique position after cutaneous-subcutaneous anesthesia using lidocaine 1%. To facilitate the intervention, first both posterolateral parts of the disc were bilaterally accessed by 17 G introducer needle (Baylis Medical Inc., Montreal, Canada). Then, two RF probes (Baylis Medical Inc., Montreal, Canada) specially designed for cooled RF practice, wherein closed circuit sterile water circulates, were fitted into the disc after they were passed through the introducers. To ensure that the probe tip was at optimal depth in the posterior annulus, the location of the probe in the tissue was controlled in the lateral and AP positions, with the radiopaque band at its tip taken as reference. The temperature = 45oC, Ramp Rate = 2.0oC/min, Time = 15 minutes.
Fig. 4. Different approach for RF intradiscal thermotherapy
Pulsed radiofrequency for L2 dorsal root ganglion for discogenic low back pain and other lumbar dorsal root ganglion for neurogenic low back pain
The PRF application procedure for L2 DRG was carried out with the patient in the prone position. The skin over the operative area was sterilized and then infiltrated with 2% lidocaine solution. Under C-arm fluoroscopic guidance, a 10-cm 22-gauge carved tip cannula with a 1-cm active tip electrode was placed toward the DRG near the intervertebral foramen. A RF generator was used. The RF electrode was positioned when sensory stimulation (50 Hz) reproduced the patient’s pain at less than 0.5 V, which indicated the location of the ganglion. The aim was to produce a tingling sensation in the dermatomal distribution of the nerve in question (Tsou et al., 2010).
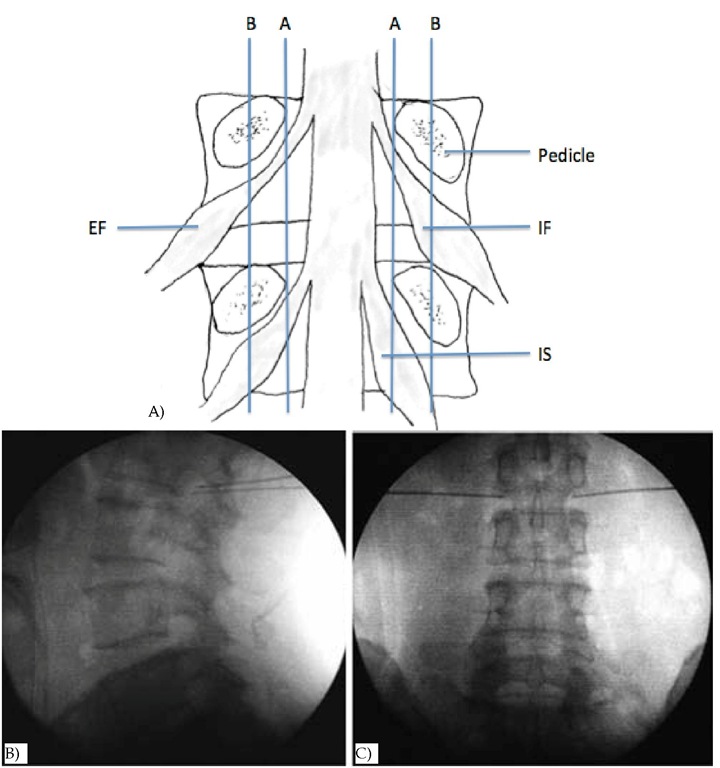
Fig. 5. DRG anatomy and ideal RF needle tips position landmark on fluoroscopy A: Positions of dorsal root ganglia (DRG) were determined by two schematic lines and classified into three types. Line A: aligning the medial borders of L4 and L5 pedicles, Line B: aligning the centers of L4 and L5 pedicles, Intraspinal type (IS) : DRG located proximal to line A, Intraforaminal type (IF) : DRG located between line A and B, Extraforaminal type (EF) : DRG located distal to line B. B & C: Lateral (B) and anteroposterior (C) radiographs showing ideal RF needle tips position of L2 DRG.
Because the DRG location has been studied, the authors divided three types of location according to the DRG and pedicle relationship (Moon et al., 2010) (Fig. 5A). The needle was advanced deeper into the intervertebral foramen until the patient felt a tingling sensation (Tsou et al., 2010) (Fig. 5B & 5C). Then 2-Hz PRF waves were applied for 120 seconds at 45 V while making sure that the electrode tip temperature did not exceed 42°C. The DRG near the intervertebral foramen of L2 root was targeted for discogenic LBP and other levels according to the radicular pain or evidence of compression noted on MRI. Motor function testing is usually not necessary with PRF because there is no risk of motor root damage with this procedure.