Trichoptera, or caddisflies, are holometabolous insects closely related to Lepidoptera, or moths. However, unlike most moths, their eggs, larvae, and pupae are usually found in or very near freshwater, and adults are aerial, usually not far from their aquatic habitats (Fig. 1) . The Trichoptera include more species than any of the other primarily aquatic orders of insects. This high species diversity is correlated with an unusual broad range of ecological specialization. Immature caddisflies occur in almost every type of freshwater habitat and on every continent except Antarctica; they are often one of the most abundant insect orders in streams and ponds. Larvae of many species use silk to construct portable cases of various shapes and materials to serve as physical protection, camouflage, or aids in respiration. Others make stationary retreats of silk for similar purposes or to serve as food-gathering structures. The variety of feeding strategies employed by caddisflies is as great as for the highly diverse freshwater Diptera, or true flies (Table I). Because of their diversity and density in most clean, freshwater ecosystems, the significance of caddisflies for processing nutrients and transferring energy is often great. Their importance as food for predators such as fish is emphasized by fly-fishing enthusiasts who imitate them with artificial lures. The different caddisfly species are variously sensitive to changes in environmental conditions, such that the diversity of the order is commonly used in part as a measure of pollution.
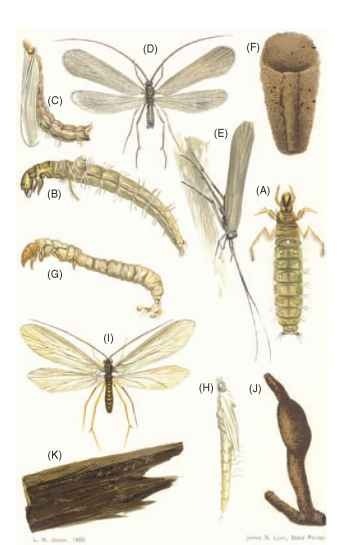
FIGURE 1 Caddisfly larvae, pupal, imagoes, cases, and eggs: (A) dorsal view of larva of Molanna cinerea, X4; (B) lateral view of larva of M. cinerea, X5; (C) lateral view of the pupa of M. cinerea, X4.5; (D) dorsal view of imago of M. cinerea, X4; (E) accustomed resting position of the imago of M. cinerea; (F) ventral view of the flat larval case of M. cinerea, X2; (G) lateral view of larva of Phylocentropus lucidus, showing the very long anal prolegs and the absence of gill filaments, X5; (H) lateral view of pupa of P. lucidus, X6; (I) dorsal view of imago of P. lucidus, X 3.5; (J) larval case of P. lucidus, tube composed of sand and silk, two-layered, enlargement near the end containing the pupa; (K) eggs laid by P. lucidus female on a stick protruding from the water in a breeding cage.
DIVERSITY AND PHYLOGENETIC RELATIONSHIPS OF TRICHOPTERA
Nearly 13,000 species of caddisflies have been described globally (Table I), with more than 1300 reported for America north of Mexico. The world species are in 613 genera in 46 families.
TABLE IExtant Families of Trichoptera, with Primary Larval Feeding Strategies, Distributions, and ApproximateNumbers of Named Species and Subspecies |
||||||||||
| Distribution11 | ||||||||||
| Family | Larval feeding strategy” | AT | AU | EP | NA | NT | OL | WP | Number of species0 | |
| Annulipalpia | ||||||||||
| Stenopsychidae | cf | X | X | X | X | X | 87 | |||
| Philopotamidae | cf | X | X | X | X | X | X | X | 1017 | |
| Hydropsychidae | cf, pe | X | X | X | X | X | X | X | 1622 | |
| Dipseudopsidae | cf | X | X | X | X | X | X | 180 | ||
| Polycentropodidae | cf, pe | X | X | X | X | X | X | X | 644 | |
| Ecnomidae | cf | X | X | X | X | X | X | X | 367 | |
| Xiphocentronidae | cg | X | X | X | X | X | X | 134 | ||
| Psychomyiidae | cg | X | X | X | X | X | X | 452 | ||
| Spicipalpia | ||||||||||
| Rhyacophilidae | pe | X | X | X | X | X | X | X | 750 | |
| Hydrobiosidae | pe | X | X | X | X | X | X | 397 | ||
| Glossosomatidae | sc | X | X | X | X | X | X | X | 581 | |
| Hydroptilidae | ph, sc, cg | X | X | X | X | X | X | X | 1929 | |
| Ptilocoloepidae | Sh | X | X | X | X | 16 | ||||
| Integripalpia | ||||||||||
| Oeconesidae | sd | X | 18 | |||||||
| Brachycentridae | cf, cg, sh | X | X | X | X | 114 | ||||
| Phryganopsychidae | sd | X | X | 3 | ||||||
| Lepidostomatidae | sd | X | X | X | X | X | X | X | 431 | |
| Kokiriidae | pe | X | X | 8 | ||||||
| Plectrotarsidae | sd | X | 5 | |||||||
| Phryganeidae | sh, pe | X | X | X | X | 87 | ||||
| Goeridae | sc, cg | X | X | X | X | X | X | 170 | ||
| Uenoidae | sc, cg | X | X | X | X | 81 | ||||
| Apataniidae | sc, cg | X | X | X | X | 201 | ||||
| Limnephilidae | sd, cg, sc | X | X | X | X | X | X | 847 | ||
| Tasimiidae | sc | X | X | 9 | ||||||
| Odontoceridae | sd | X | X | X | X | X | X | 119 | ||
| Atriplectididae | sd | X | X | X | 4 | |||||
| Philorheithridae | pe | X | X | 26 | ||||||
| Molannidae | sc, cg, pe | X | X | X | X | 38 | ||||
| Calamoceratidae | sd, sc, | X | X | X | X | X | X | X | 161 | |
| Leptoceridae | cg, sh, pe | X | X | X | X | X | X | X | 1813 | |
| Sericostomatidae | sd | X | X | X | X | X | X | 98 | ||
| Beraeidae | cg | X | X | X | X | 51 | ||||
| Anomalopsychidae | sc | X | 22 | |||||||
| Helicopsychidae | sc | X | X | X | X | X | X | X | 239 | |
| Chathamiidae | sh | X | 5 | |||||||
| Helicophidae | cg, sh | X | X | 34 | ||||||
| Calocidae | cg | X | 23 | |||||||
| Conoesucidae | sc, sd, sh | X | 42 | |||||||
| Antipodoeciidae | sc | X | 1 | |||||||
| Barbarochthonidae | sh, sc | X | 1 | |||||||
| Hydrosalpingidae | sc, sd, sh | X | 1 | |||||||
| Limnocentropodidae | pe | X | X | 14 | ||||||
| Petrothrincidae | sc | X | 8 | |||||||
| Pisuliidae | sd | X | 16 | |||||||
| Rossianidae | sc, sh | X | 2 | |||||||
^Abbreviations: cf, collector-filterers; cg, collector-gatherers; pe, predator-engulfers; ph, piercer-herbivores; pp, predator-piercers; sc, scrapers; sd, shredder-detritivores; sh, shredder-herbivores. [From Merritt et al., 2008. Some larval feeding strategy data from M. Winterbourne, University of Canterbury, Christchurch, New Zealand and F. C. de Moor, Albany Museum, Grahamstown, South Africa.]
bAbbreviations: AT, Afrotropical; AU, Australasian; EP, East Palearctic; NA, Nearctic; NT, Neotropical; OL, Oriental; WP, West Palearctic. cThe total number of these known species and subspecies is 12,868.
The most species have been described in the microcaddisfly family, Hydroptilidae, with over 1900 species. Other well-represented families globally include the long-horned caddisflies (Leptoceridae, >1800 species), the common netspinner caddisflies (Hydropsychidae, >1600 species), and the northern caddisflies (Limnephilidae, almost 900 species). The highest known species diversity and the greatest density of species occur in the Oriental biogeographical region (>4400 species, with 1.6 species per kilohectare).
The Trichoptera and Lepidoptera are generally considered to be sister orders, together constituting the Amphiesmenoptera, with earliest fossils dating from the Permian. The families of tube-case-making caddisflies are generally considered to constitute a monophyletic suborder Integripalpia and the net-spinning caddisfly families a monophyletic suborder Annulipalpia (Fig. 2) . The relationships of the remaining caddisfly families (Glossosomatidae, Hydrobiosidae, Hydroptilidae, and Rhyacophilidae, collectively the “Spicipalpia”) are yet unknown but are apparently near the base of the caddisfly phylogeny. The phylogenetic relationships among genera and species of caddisflies have been studied for most extant families, mostly with morphological characters visible with a light microscope. The phylog-eny of Trichoptera species continues to be investigated at all categorical scales by Kjer and others, using both morphological and molecular evidence and computer-managed algorithms. These hypothetical relationships have proven useful in numerous comparative studies, suggesting hypotheses for research of case- and retreat-making behaviors, feeding strategies, historical biogeography, mating behaviors, and other aspects of caddisfly biology.
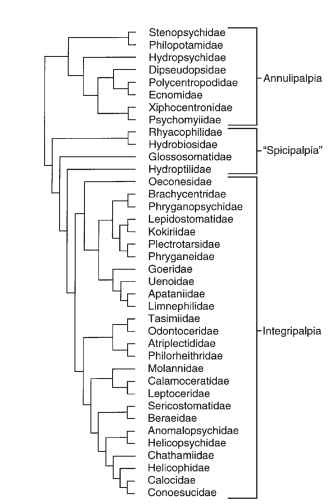
FIGURE 2 Phylogeny of extant families of Trichoptera; families not analyzed by Kjer et al. include Antipodoeciidae, Barbarochthonidae, Hydrosalpingidae, Limnocentropodidae, Petrothrincidae, Pisuliidae, and Rossianidae.
Triassic fossils of species of Philopotamidae, Prorhyacophilidae, and Necrotauliidae are thought to represent the oldest known Trichoptera. The family Philopotamidae includes modern species, but the other two families are extinct.
LIFE HISTORIES OF TRICHOPTERA Life Cycles
Among the relatively few species of caddisflies for which life histories are known, most exhibit a univoltine, or 1-year, life cycle, with some species having more than one generation per year and some with one generation every 2 or 3 years. In general, longer life cycles are found in the higher latitudes and shorter life cycles closer to the equator. A few species have been shown to have more than one cohort in the same locality, with different segments of the same population having their life cycles synchronized apart from the synchrony of one or more other segments. The time of the year during which particular phases of the life cycle occur is distinctive for the species, with most species reaching adulthood in temperate regions during the warmer months of the year.
Eggs are embedded in a sticky, gelatinous polysaccharide called spumalin, forming an egg mass. Round or elliptical eggs are generally laid in egg masses of particular shapes with 12-636 eggs reported per egg mass and with a single female depositing one to several egg masses. Egg masses may be spherical, pyramidal, butterfly shaped, or some other configuration, or they may be arranged in a flat spiral or as a string of beads. Because of the spumalin, little is known about the surface structure of caddisfly eggs. Eggs may diapause for several months before larvae hatch, especially during winter, but larvae generally hatch within a few days depending on temperature. If the egg mass is out of water, the first instars of some species may remain in the spumalin matrix until it is inundated; for other species whose egg masses are laid on plants or rocks overhanging the water, young larvae drip out of the spumalin into the water.
Larvae
Larvae usually undergo five instars before pupation, although a few species have a generally indeterminate number of instars greater than five. The larval stage usually is the longest stage of the life cycle, with development completed during a period of 2 months to nearly 2 years. The shape of the larva differs usually with the family or genus and depends in part on the habits and feeding strategy peculiar to that taxon. Eruciform larvae, typical of the Integripalpia, are cylindrical, with hypognathous mouthparts oriented at a right angle with the body axis and with the posterior end of the abdomen blunt and having thick anal prolegs fused with segment IX; these live in portable tubular cases and feed generally as shredders or scrapers or collector-gatherers.
Campodeiform larvae, typical of the Annulipalpia and unplaced primitive families, have a tapered shape anteriorly and posteriorly, with prognathous mouthparts nearly aligned with the body axis; the posterior end of the abdomen is slender, and there are slender, independent or semi-independent anal prolegs. These larvae live in silk retreats or roam freely in search of food. Larvae of microcaddisflies (family Hydroptilidae) typically undergo hypermetamorphosis, with the first four instars campodeiform and free-living and the fifth instar with a much-enlarged abdomen and living in a purse case.
Pupae
The last instar builds a shelter or modifies the larval case to serve as a shelter. Modification of a larval case usually means simply sealing the ends with silk closure membranes that are perforated to allow movement of water through the shelter. Larvae may then line the shelter with silk; a few families (Glossosomatidae, Hydrobiosidae, Rhyacophilidae) spin a semipermeable cocoon inside the shelter. The larva may then diapause for a few months as a prepupa inside the shelter, but usually it proceeds immediately to molt into the pupal stage, usually retaining the exuviae of the last instar inside the shelter. The pupal appendages are not fused with the body, as they are in some Lepidoptera and Diptera, but are merely folded against the body, with the wings and antennae wrapped around the sides and venter of the body.
Special structures of the pupa include long mandibles for keeping debris from blocking anterior closure membrane perforations and for cutting the closure membrane or cocoon at the time of emergence, setose caudal processes for keeping debris from blocking posterior closure membrane perforations, and small plates of hooks dorsally on the abdomen for maneuvering the pupa inside the silk-lined shelter or cocoon. Respiration is assisted by undulating movements of the pupa, forcing water through the shelter and cocoon from anterior to posterior ends. The pupa usually completes development to the adult stage in 2 or 3 weeks.
Emergence
When the pupa is ready to transform to the adult stage, it uses its long mandibles to cut through the shelter’s anterior closure membrane, swims to the water surface, and emerges from the pupal exuviae at the surface in open water or on some emergent object. In the molt from pupa to adult, the period between apolysis and ecdysis is unusually long, allowing body sclerites to complete much of their sclerotization before the adult emerges. In this way, the adult cad-disfly is able to fly quickly from the surface of the water, away from predatory fish, usually completing the escape from the shelter and the water surface in less than a minute. The teneral adult then rests on shoreside vegetation or rock until sclerotization is complete.
Adult
Nearly all adult caddisflies are capable of flight, with only a few species having short, flightless wings. They are secretive, hiding among shoreside vegetation or rocks most of the time, with periods of activity in the day or night specific for the different species. Adults of different species live for a few days to several months. Those that have long adult lives have an especially well-developed haustellum for imbibing liquids; apparently they ingest water or nectar for sustenance. Mating behavior for some species is mediated by sexual pheromones, with females emitting odors that attract males. Males of some day-flying species also exhibit distinctive flight patterns or swarms that attract females. Mating is accomplished while standing on shoreside substrate in an end-to-end orientation with tips of the wings overlapping; the male inferior appendages, or “claspers,” hold the end of the female abdomen while he inserts his phallus and releases a spermato-phore into her spermatheca. The female then lays her egg mass. It may be laid on a rock or vegetation overhanging the water, from which first instars may drip into the water upon hatching. It may be laid on underwater substrates by a female that crawls or swims beneath the water surface. A few species may lay an egg mass in a protected spot in a dry depression that will later be filled with water.
HABITATS AND DISTRIBUTION OF TRICHOPTERA
Habitats
Immature stages of caddisflies may live in a wide range of habitats with fast to slow current speed or in standing water, but the habitat preferences of the individual caddisfly species usually are quite restricted. Most species require relatively clean, cool water with high concentration of dissolved oxygen. In these habitats, larvae may be found in accumulations of dead leaves and sticks, on or in woody debris, on the tops or bottoms or sides of stones, burrowing in sand or silt, in the crevices among gravel, on submerged or floating parts of living plants, or on other relatively stable debris. A few species complete their entire life cycles away from water, with immature stages crawling among leaves on the forest floor or clinging to vertical rock faces. Immature stages of a few species develop in brackish water, and those of species of the family Chathamiidae apparently all develop only in ocean surf on the shores of New Zealand, at least one of them in a complex mutualistic relationship with starfish.
Distribution
Caddisflies are distributed throughout the world, with species known from every continent except Antarctica. The greatest known species diversity is in the Oriental biogeographic region, where the density of species per hectare is nearly twice that of the next most diverse region.
CASE AND RETREAT MAKING BY TRICHOPTERA
Case Making
Caddisfly larvae have long been appreciated for the beautiful and complex cases that many of them build. These usually are not attached to a substrate but are carried by the larva as it crawls or swims in its habitat. Larvae build cases from a wide range of mineral and plant materials, with the type of material and the shape of the case often recognizable in the field for the genus or even for the species. Mineral building materials may include sand grains or small stones, which may be all of one size or may be of two or more sizes organized in a species- or genus-specific pattern, for example with larger “ballast” stones laterally. The larva selects a mineral particle of preferred size and shape and applies it to the anterior edge of the case with silk extruded from the spinneret in its mouth. Examples of plant building materials include living or dead pieces of leaves or wood that have been shaped by the larva before their attachment to the anterior edge of the case with silk. The preferred plant material may be very specific, such as algae, mosses, liverworts, or rootlets or leaves of particular vascular plants growing in the water, or pine needles, sticks, or leaves that fall from shoreside vegetation. These materials may be oriented longitudinally or horizontally; longitudinal materials may be organized in rings or in a spiral; horizontal materials may be interlocked at corners of a two-, three-, or four-sided case or may be wrapped in a cylindrical case. The larvae of a few species make their cases by hollowing the axis from a stick or piece of wood. Some cases are made entirely from silk or may incorporate pieces of freshwater sponge or abandoned shells of freshwater mollusks. At least one marine species builds its case with bits of coral.
The cases of caddisflies are often classified as tube cases (most Integripalpia), saddle cases or tortoise cases (Glossosomatidae), or purse cases (Hydroptilidae). A tube case is more or less cylindrical,surrounding the larva at approximately the same distance from all sides, although the shape of the case, especially externally, may vary greatly from one species to another. Thus, a tube case may be externally cylindrical or four-sided or three-sided or flattened; it may have lateral and/or anterodorsal flanges; or it may be straight or curved or coiled into a tight spiral like a snail shell. A tube case almost always has recognizable anterior and posterior ends. Pupation occurs within the tube case after it has been fastened to stationary substrate and the ends have been sealed with silk membranes (except for holes left for water circulation).
A saddle case is more or less domelike, oval on its flat side, always of mineral materials, sometimes with larger stones laterally. A transverse strap of fine sand grains connects the longer sides of the oval ventrally, beneath the larva, leaving anterior and posterior openings on the ventral side from which the head and anus of the larva protrude interchangeably. This ventral strap is removed and the dome is fastened to stable substrate by the larva as it prepares to pupate under the dome.
A purse case typically consists of two flat sheets of silk (often including sand or algae) that have been sewn together at their edges, leaving the ends open for the head and anus to protrude interchangeably. Purse cases sometimes are cemented to the substrate. Pupation occurs within the purse case after the case has been fastened to stationary substrate and the ends have been sealed with silk membranes.
Retreat Making
About half the species of caddisflies do not build cases, but instead spin silken retreats that are stationary, fastened to the substrate. These retreats often are modified to assist with capturing food, usually by filtering it from moving water. The shape of a retreat usually is characteristic of a family or genus and may appear on wood or stone substrate—for example, as a flat sheet over a shallow depression on the substrate; a long, sinuate tunnel or a short covering that incorporates bits of mineral or plant material; a fingerlike net on the undersides of stones; or a bag of silk suspended from substrate in the current. Some species construct a silk-lined vertical tube in sandy soil. Pupation occurs either in the silken retreat or in a special pupation chamber constructed of silk and plant or mineral substrate by the larva.
Free-Living Larvae
Larvae of species of Rhyacophilidae and Hydrobiosidae are free-living predators, roaming about the substrate in search of prey. Pupation occurs under a domelike pupation chamber constructed of silk and plant or mineral substrate by the larva.
FEEDING STRATEGIES OF TRICHOPTERA
Although some long-lived adults may imbibe water or nectar, most nutrients are acquired by larvae. Larvae feed in a variety of ways, with a greater diversity of feeding strategies than any other group of insects having a comparable number of species. Early instars of most species and late instars of many species are collector-gatherers, picking small bits of loose organic material from the substrate. Many other species are collector-filterers, using silken nets (usually) or hairy legs to strain small bits of organic matter from moving water. Shredding herbivores chew pieces of leaves of living plants, often using some of the same leaves in their cases. Shredding detritivores gouge rotting wood or cut pieces of dead leaves that have been “conditioned” by fungi and bacteria, getting most of their nutrition from digestion of the fungi and bacteria. Scrapers graze on the algae, fungi,and associated organic material (“periphyton”) that is attached to the surfaces of stones and plant material exposed to sunlight. Predators either chase their prey or lie in wait for it to wander nearby; prey items typically include other insects, microcrustaceans, and annelids; the predators either swallow their prey whole or they chew them in bits. Some caddisfly larvae may eat the flesh of dead animals, such as dead fish, as these become available. Some species of long-horned caddisflies feed facultatively or obligatorily on freshwater sponge, ingesting whole pieces of the sponge, including the spicules, which accumulate in the gut.
IMPORTANCE OF TRICHOPTERA
Caddisflies are one of the major groups of macroinvertebrates in freshwater ecosystems both in terms of species diversity and of density, especially in relatively unpolluted waterways. For this reason, they are significant contributors in the processing of nutrients. On one hand, collecting-gathering and collecting-filtering and scraping caddisflies help concentrate the nutrients of fine particulate organic matter into their own bodies, making the nutrients available to invertebrate and vertebrate predators in the food web. On the other hand, shredding herbivores and shredding detritivores and predators help to break coarse organic materials into small particles, including feces, that can then be used by many animals that are able to ingest only fine bits of organic material.
Food Resources
Because of the many different feeding strategies and habitat preferences of this diverse order, nearly every conceivable food resource is processed by caddisflies. Because their populations are so large, they process significant amounts of these resources for the benefit of the other animals in the ecosystem. There is even evidence that scraping caddisflies help to increase the production rate of the attached algae on which they feed, much as mowing the grass in a lawn or pruning new growth of fruit trees stimulates increased growth and production in those plants. The role of caddisflies in the food web is appreciated very well by sport fishing enthusiasts, who tie imitations of larval, pupal, and adult caddisflies on hooks to entice their game fish to bite a hook. The more those who fish learn about the species diversity, behavior, and biology of caddisflies, the more likely they are to succeed in tricking the fish to take the hook.
Pollution Tolerance
Although caddisflies generally will not tolerate even moderate levels of pollution, the range of tolerances is wide among the various species of caddisflies. For this reason and because of the usual high species diversity and density of caddisflies in unpolluted surface waters, communities of Trichoptera and other macroinvertebrates are often used to detect the presence of pollution. The occurrence of several of the less-tolerant species and high densities of large numbers of species of caddisflies suggest that the water is relatively unpolluted. Pollution may be detected with this technique more reliably and more cheaply than with chemical analyses. Once it has been established that a given waterway is polluted, follow-up analyses may then be attempted to discover the specific polluting substances or microorganisms and their concentrations. Finally, equipped with those data, land managers, engineers, economists, politicians, and other responsible decision makers may be better able to determine appropriate mitigating measures to reduce or eliminate the pollution.
