Mouthparts
The mouthparts of insects are structures surrounding the mouth that are involved in the mechanics of feeding and processing and manipulating the food so that it can be ingested. Although functionally equivalent to the jaws of vertebrates, they lie outside the mouth, not within a buccal cavity. Good basic accounts of insect mouthpart structure are to be found in most text topics of entomology. The aim of this article is to supplement these basic accounts by briefly considering some of the variations associated with different feeding habits and different types of food. It also gives some information on the functioning of the mouthparts.
BITING AND CHEWING INSECTS
Insect mouthparts are derived from the appendages of four of the segments forming the insect head. They surround the mouth and are external to it, unlike the condition in vertebrates in which the teeth are within the oral cavity. The basic segmental character of the mouthparts is most apparent in insects that bite off fragments of food and then chew it before ingesting it (Fig. 1). Insects that do this are said to be “mandibulate” because the mandibles are relatively unmodified compared with those of fluid-feeding insects (see below). These are also commonly called biting mouthparts, although there is some risk of confusion with blood-sucking insects, such as mosquitoes, which bite! In this article the latter are distinguished as “piercing.” The mandibulate arrangement occurs in the primitively wingless insects (Apterygota), in the cockroaches and grasshoppers and their allies, in larval and adult beetles (Coleoptera) and most Hymenoptera, and in caterpillars (larval Lepidoptera), among the more advanced groups of insects.
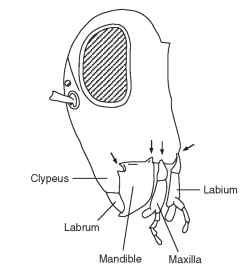
FIGURE 1 A lateral view of the head of a grasshopper showing the segmental arrangement of the mouthparts: labrum, mandible, maxilla, and labium. Arrows show the points of articulation (condyles) with the head capsule. The mandible has two condyles (dicondylic), the maxilla only one, and the labium one on each side.
Immediately in front of the mouth is the labrum formed from the fusion of the appendages on either side of the labral segment. It comprises a flat sclerotized plate of cuticle continuous with the cuticle of the front of the head (clypeus). Its inner side (toward the mouth) is known as the epipharynx, and it is formed from membranous cuticle-bearing tracts of noninnervated hairs, all pointing toward the mouth. In grasshoppers, and probably in other insects with similar mouth-parts, the hairs are easily wetted, whereas the other parts of the cuticle are water repellent. The hydrophilic hairs may serve to direct the flow of fluid from the food toward the mouth and also to groups of contact chemoreceptors (taste receptors) that occur just outside the mouth. Contact chemoreceptors also often occur along the distal edge of the labrum. At rest, the labrum presses back on the mandibles, which are immediately behind it, being held in this position by a rubber-like protein, called resilin, in its connection with the clypeus.
The mandibles, one on each side, are hinged to the head capsule by one or two condyles. Archaeognatha have only one condyle (monocondylic), whereas Thysanura and all mandibulate pterygote insects have two (dicondylic). The change from one to two condyles represents a considerable evolutionary advance because it gives the mandibles a much firmer base and so facilitates feeding on hard materials. The mandibles of the two sides swing transversely to meet below or in front of the mouth, depending on the orientation of the head, and are opened and closed by a pair of muscles, one inserted on either side of the axis of mandibular attachment at the condyles. The opener muscle is called the abductor, whereas the closer is the adductor. The latter is the larger of the two because it provides the force necessary to bite into or through material. Both muscles arise on the cuticle at the top of the head and, in grasshoppers and caterpillars, the head capsule grows bigger to accommodate the increased size of the adductor muscle if the insect feeds on tough food.
The two mandibles are asymmetrical so that where they meet in the midline the cusps on the biting surface of the two sides fit between each other (Fig. 2 ) . These cusps are extremely hard. In addition to being sclerotized like the hard cuticle elsewhere in the body, their cuticle contains zinc or manganese or, occasionally, iron, which are assumed to contribute to the hardness. The form of the biting cusps varies from species to species in relation to feeding habits in a way that, superficially, is comparable with the adaptations in the jaws of mammals. Predaceous tettigoniids, for example, have a sharply pointed cusp dis-tally and powerful blade-like cusps more proximally that have some resemblance to the canine and carnassial teeth of carnivorous mammals and presumably serve similar functions of grasping and tearing the prey. Among grasshoppers, species feeding on soft, broad-leaved plants have small, sharply pointed cusps that cut the food into very small fragments. Grass feeders, on the other hand, have very long, chisel-edged incisor cusps distally with short, flattened molar cusps proximaUy, which can superficially be compared with the chisel-shaped incisors and grinding molars of mammalian herbivores. Other insects also exhibit food-related modifications of the mandibles. The cusps become worn down with use, especially if the insect is feeding on hard foods, and there is some evidence that this wearing down reduces the rate of food intake. The cusps can be renewed only at a molt, when new cuticle is formed.
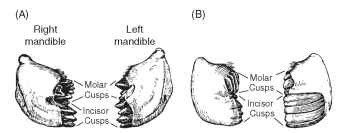
FIGURE 2 Mandibulate mouthparts. Mandibles, seen from in front with the labrum removed, of grasshoppers with different feeding habits. Notice that the mandibles of the two sides are asymmetrical. (A) A grasshopper that feeds on soft, broad-leaved plants. (B) A grasshopper that feeds on grasses.
In insects eating food that requires special treatment during inges-tion, the mandibles may become highly modified. An example occurs in the larvae of pergine sawflies (Fig. 3). These Australian insects feed on Eucalyptus and related trees, the leaves of which are rich in essential oils. The insects sequester the oils in a diverticulum of the foregut and use them for defense. The mandibles are apparently adapted for separating the oils from the leaf tissue. Sticking out from the center
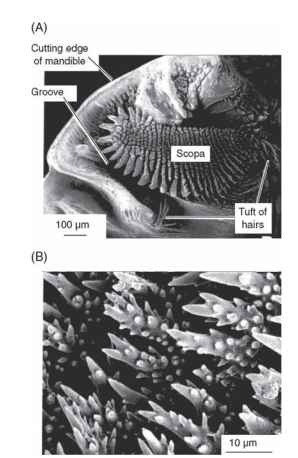
FIGURE 3 Mandibulate mouthparts. Specialized mandible of a pergid sawfly larva. These insects feed on Eucalyptus leaves containing large quantities of essential oils. The oils are apparently squeezed from the leaf tissue by the action of the scopa mandibularis (scopa) and conducted along the groove to a pharyngeal diverticulum where they are stored. (A) Surface view of the biting face of the right mandible showing the rows of spines forming the scopa mandibularis. (B) Spines of the scopa mandibularis, which presumably abrade the leaf surface to release the oils.
of the mandible is a structure called the scopa mandibularis. It is covered by rows of pointed setae and these, perhaps by scraping and shredding the leaves, seem to be involved in extracting the oils.
In the midline, immediately behind the mouth and probably also derived from the mandibular segment, is the hypopharynx. This structure is a lobe of mostly membranous cuticle but with rods of sclerotized cuticle to which muscles are attached. Like the epiphar-ynx, it bears tracts of hairs pointing toward the mouth and these hairs probably help to move food toward the mouth as the hypopharynx is moved by its muscles.
Behind the mandibles are the maxillae, one on each side of the head. Each maxilla articulates with the head capsule by a single con-dyle so that it is extremely mobile. This high degree of movement allows the maxillae to manipulate food between the mandibles and move it toward the mouth. The laciniae at the distal ends of the maxillae are especially important for this and they are usually curved and pointed with the tip hardened like the mandibles. The maxillary palps are leg-like structures often with three to five segments and they have an important sensory function. At the tip of each palp is an array of contact chemoreceptors; in a large grasshopper there may be as many as 400 chemosensilla on the tip of each palp. These receptors have an important role in food selection. Grasshoppers drum on a leaf surface with the palps before accepting or rejecting it as food, and they continue to drum at intervals during feeding. Chemoreceptors are also present on the galea, a distal lobe of the maxilla immediately lateral to the lacinea.
The labium is essentially similar in structure to the maxillae but with the appendages of the two sides fused together in the midline behind the hypopharynx. There is a single articulation with the head capsule on each side, which allows the labium to swing beneath the head in the vertical plane of the body. It provides a scoop that prevents food from spreading backward from between the mandibles. As with the maxilla there are two terminal extensions on each side, known as the glossa and paraglossa. There is also a leg-like labial palp with chemoreceptors at the tip.
The labium is uniquely developed in larvae of dragonflies and damselflies, forming their prey capture equipment. The form is basically similar to the labium of other insects except that the basal parts are lengthened and the palps are claw-like. It is sometimes called a labial mask because the distal parts cover the lower part of the face when the labium is folded beneath the head. The mask can be suddenly extended by hemolymph pressure, enabling the larva to capture prey a little distance in front of it without moving its body.
FLUID-FEEDING INSECTS
Many insects feed on liquid food and their mouthparts are modified to form a tube through which fluid can be drawn into the mouth and, often, another tube through which saliva is injected into the food so that it is digested to some extent before being ingested. In most fluid-feeding insects the basic segmental arrangement and appendicular form of the mouthparts are no longer obvious, but some predaceous larvae that feed on the body fluids of their hosts are mandibulate, with mandibles resembling those of insects feeding on solid food. These are larvae of lacewings and ant lions (Neuroptera), glowworms (Lampyridae), and dytiscid beetles. In all of them, the mandibles are sickle shaped with a groove along the inner edge. In the beetles, the two sides of the groove arch over to meet, or almost meet, so that a tube is formed. In ant lions, the lacinea of the maxilla is also sickle shaped and it fits in the mandibular groove to form an enclosed canal. These insects can pump the fluid contents of their prey into the foregut through the tubes.
In other fluid-feeding insects, the basic segmental arrangement of the mouthparts is not apparent and in many insects the mouthparts themselves are drawn out into long, slender structures called stylets. The food and salivary canals are formed in different ways in different insect groups. In Hemiptera, both canals are formed between the styliform maxillae, which interlock by a tongue-and-groove mechanism that permits them to slide lengthwise with respect to each other but prevents them from coming apart (Fig. 4) . They are supported in a groove along the anterior margin of the elongate labium, which is often referred to as the rostrum. The food canal is formed between the maxillary galeae in Lepidoptera, but here the two sides are linked by a series of cuticular hooks and plates that hold the two sides together while allowing them to coil up beneath the head when not in use (Fig. 5 ). This device makes it possible for some lepidopterans to have an extremely long tongue, which would not be possible if the insect were unable to
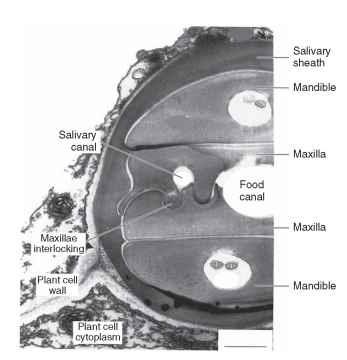
FIGURE 4 Piercing and sucking mouthparts of a hemipteran Electron micrograph of a transverse section through part of the stylei bundle of an aphid within a leaf. Notice how the maxillary stylet interlock to form the food canal and the salivary canal. The stylets are surrounded by a sheath of solidified saliva that is produced as the) penetrate the plant. The stylets are within the cell wall, which i seen in its normal form at lower left.
coil it. The longest examples are in the hawk moths, the Sphingidae. Many of these have a tongue 30 mm or more in length, but one species, Cocytius cluentis, from South America has a tongue 250 mm long! Lepidopterans have no salivary canal in the tongue because the nectar on which they feed does not require digestion before being ingested. Each galea contains an extension of the hemocoel and the proboscis is uncoiled by an increase in pressure generated at the base of each galea. A series of short muscles extends across the galea and these muscles are involved in coiling the proboscis beneath the head. There are contact chemoreceptors at the tips of the galeae and the axons from the sensory receptor cells combine to form a nerve, which also contains motor axons to the muscles, running the length of each galea. Oxygen is supplied to these tissues via a longitudinal trachea.
Among the flies (Diptera), the feeding canal is a groove along the underside of the long labrum, closed behind by the other mouth-parts, whereas the salivary canal is a narrow tube running through the styliform hypopharynx. The labium forms a sheath that encloses the stylets formed by the other mouthparts and is called the haustellum in the higher Diptera. In addition, in cyclorraphous flies the distal part of the labium is extended to form a flattened membranous lobe called the labellum. It is conspicuous in house flies and blowflies (Fig. 6). The ventral surface of each labellum is invaginated to form a series of channels that collect together medially where they make contact with the food canal in the labrum. The walls of the channels are supported by a series of incomplete rings of sclerotized cuticle. These rings prevent the channels from collapsing when suction is exerted by the
![Sucking mouthparts of a butterfly. (A) Proboscis coiled beneath the head. The labial palp on the left (near) side has been removed. Abbreviations: c, clypeus; ce, compound eye; lp, labial palp; pr, proboscis. [Reprinted from Krenn, H. W., and Penz, C. M. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen-feeding behavior. Int. J. Insect Morphol. Embryol. 27, 301-309, © 1998, with permission from Elsevier Science.] (B) Transverse section through the proboscis near the base. The galea of either side fit together to form the food canal. Each galea is blood filled and contains a nerve and trachea running the full length of the proboscis and short muscles (seen in oblique cross sections in the diagram) that run obliquely across the galea and are involved in coiling the proboscis. Sucking mouthparts of a butterfly. (A) Proboscis coiled beneath the head. The labial palp on the left (near) side has been removed. Abbreviations: c, clypeus; ce, compound eye; lp, labial palp; pr, proboscis. [Reprinted from Krenn, H. W., and Penz, C. M. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen-feeding behavior. Int. J. Insect Morphol. Embryol. 27, 301-309, © 1998, with permission from Elsevier Science.] (B) Transverse section through the proboscis near the base. The galea of either side fit together to form the food canal. Each galea is blood filled and contains a nerve and trachea running the full length of the proboscis and short muscles (seen in oblique cross sections in the diagram) that run obliquely across the galea and are involved in coiling the proboscis.](http://lh3.ggpht.com/_X6JnoL0U4BY/S8HbnXDgLxI/AAAAAAAAYVU/Fifzf4-jtKg/tmp95_thumb_thumb.jpg?imgmax=800)
FIGURE 5 Sucking mouthparts of a butterfly. (A) Proboscis coiled beneath the head. The labial palp on the left (near) side has been removed. Abbreviations: c, clypeus; ce, compound eye; lp, labial palp; pr, proboscis. [Reprinted from Krenn, H. W., and Penz, C. M. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen-feeding behavior. Int. J. Insect Morphol. Embryol. 27, 301-309, © 1998, with permission from Elsevier Science.] (B) Transverse section through the proboscis near the base. The galea of either side fit together to form the food canal. Each galea is blood filled and contains a nerve and trachea running the full length of the proboscis and short muscles (seen in oblique cross sections in the diagram) that run obliquely across the galea and are involved in coiling the proboscis.
pumps in the head and give the channels an appearance that is superficially like that of tracheae and so they are called pseudotracheae. The labellum, with the pseudotracheae, enables the fly to draw fluids from a relatively large surface. The channels open to the exterior via a narrow groove that is closed off during feeding except for occasional openings through which fluid can pass freely. Fleas have the food (blood) channel between a highly developed epipharynx and the two maxillae. There are two salivary canals, one in each maxilla.
The mouthparts of bees are unusual among the fluid-feeding insects. They retain normal mandibles that are used for wax and pollen manipulation, but are not involved in nectar feeding. The other mouth-parts retain some semblance of the appearance in biting and chewing insects, but are elongate. The two glossae (parts of the labium) are fused together to form an elongate tongue with an open gutter posteriorly. The glossal tongue is surrounded by the lengthened and flattened galeae (of the maxillae) and labial palps. The food canal is formed by the space between the glossal tongue and the other components.
Lepidoptera, bees, and some flies feed from fluids, often nectar, that is present on a surface, but other fluid-feeding insects obtain their food from within plants or other animals and so must pierce the host tissues before being able to feed. This is true of all Hemiptera, fleas, and some flies. In Hemiptera, the mandibular stylets are the main piercing structures. The relatively stout labium does not enter the wound, but folds up beneath the insect as the mandibles and maxillae penetrate deeper into the host tissues. The stylets of aphids and coccids are very flexible and usually follow intercellular pathways that may be quite tortuous. The watery saliva of aphids contains a pectinase that degrades the pectin of the cell walls and facilitates movement of the stylets.
Among blood-sucking flies, the maxillae are the primary piercing organs of female mosquitoes (Fig. 7). They have recurved teeth
![Sucking mouthparts of a blowfly. (A) Feeding by suction. With the labellar lobes spread flat on a surface, the openings of the pseu-dotracheae are brought into contact with fluid on the surface. Suction exerted by the pharyngeal pump draws liquid up the food canal and so through the pseudotracheal openings and along the pseudotracheae. Notice that the prestomal teeth are not exposed. Arrows show the direction of flow. (B) Feeding by rasping. By pulling back the labellar lobes, the prestomal teeth can be brought into contact with the substrate and are used to rasp at solid food. [A and B reproduced with permission from Cambridge University Press, from Graham-Smith, G. S. (1930). Further observations on the anatomy and function of the proboscis of the blowfly. Calliphora erythrocephala L. Parasitology 22, 47-115.] (C) View of the ventral surface of the labellum showing the arrangement of pseudotracheae. The smaller branches join with major collecting trunks, which are functionally connected to the food canal. [Reproduced with permission from Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc., from Wilczek, M. (1967). The distribution and neuroanatomy of the labellar sense organs of the blowly Phormia regina Meigen. J. Morphol. 122, 175-201.] (D) Ventral view of the labellum with the prestomal teeth everted for rasping. Abbreviations: c, openings of collecting channels into which the pseudotracheae open; p, pseudotracheae; t, prestomal teeth. Sucking mouthparts of a blowfly. (A) Feeding by suction. With the labellar lobes spread flat on a surface, the openings of the pseu-dotracheae are brought into contact with fluid on the surface. Suction exerted by the pharyngeal pump draws liquid up the food canal and so through the pseudotracheal openings and along the pseudotracheae. Notice that the prestomal teeth are not exposed. Arrows show the direction of flow. (B) Feeding by rasping. By pulling back the labellar lobes, the prestomal teeth can be brought into contact with the substrate and are used to rasp at solid food. [A and B reproduced with permission from Cambridge University Press, from Graham-Smith, G. S. (1930). Further observations on the anatomy and function of the proboscis of the blowfly. Calliphora erythrocephala L. Parasitology 22, 47-115.] (C) View of the ventral surface of the labellum showing the arrangement of pseudotracheae. The smaller branches join with major collecting trunks, which are functionally connected to the food canal. [Reproduced with permission from Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc., from Wilczek, M. (1967). The distribution and neuroanatomy of the labellar sense organs of the blowly Phormia regina Meigen. J. Morphol. 122, 175-201.] (D) Ventral view of the labellum with the prestomal teeth everted for rasping. Abbreviations: c, openings of collecting channels into which the pseudotracheae open; p, pseudotracheae; t, prestomal teeth.](http://lh4.ggpht.com/_X6JnoL0U4BY/S8HbplUcMDI/AAAAAAAAYVc/kO-1bHQHoHs/tmp96_thumb_thumb.jpg?imgmax=800)
FIGURE 6 Sucking mouthparts of a blowfly. (A) Feeding by suction. With the labellar lobes spread flat on a surface, the openings of the pseu-dotracheae are brought into contact with fluid on the surface. Suction exerted by the pharyngeal pump draws liquid up the food canal and so through the pseudotracheal openings and along the pseudotracheae. Notice that the prestomal teeth are not exposed. Arrows show the direction of flow. (B) Feeding by rasping. By pulling back the labellar lobes, the prestomal teeth can be brought into contact with the substrate and are used to rasp at solid food. [A and B reproduced with permission from Cambridge University Press, from Graham-Smith, G. S. (1930). Further observations on the anatomy and function of the proboscis of the blowfly. Calliphora erythrocephala L. Parasitology 22, 47-115.] (C) View of the ventral surface of the labellum showing the arrangement of pseudotracheae. The smaller branches join with major collecting trunks, which are functionally connected to the food canal. [Reproduced with permission from Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc., from Wilczek, M. (1967). The distribution and neuroanatomy of the labellar sense organs of the blowly Phormia regina Meigen. J. Morphol. 122, 175-201.] (D) Ventral view of the labellum with the prestomal teeth everted for rasping. Abbreviations: c, openings of collecting channels into which the pseudotracheae open; p, pseudotracheae; t, prestomal teeth.
distally that probably anchor the stylets in position in the wound so that when the retractor muscles contract they pull down toward the host skin, pushing the labrum into the wound at the same time. Male mosquitoes do not feed on blood and, in many species, the piercing stylets are greatly reduced. Horse flies and deer flies (Tabanidae) have a completely different mechanism. Their somewhat elongate mandibles are articulated to the head capsule so that they move transversely, like the mandibles of biting and chewing insects. This scissor-like motion cuts through the skin of the host and the labrum and maxillae are forced into the wound. The labium does not enter the wound and blood is taken directly into the food canal on the inner side of the labrum. Tsetse flies (Glossina) and stable flies (Stomoxys) penetrate the host tissues by a rasping movement of prestomal cutic-ular teeth on the labellar lobes (Fig. 8). House flies have similar, but much smaller teeth that they may use to rasp the surface of solid food, but in their blood-sucking relatives the teeth are stronger and are accompanied by banks of cuticular spines that form rasps. When these flies feed, the teeth and rasps are rapidly rotated round the tip
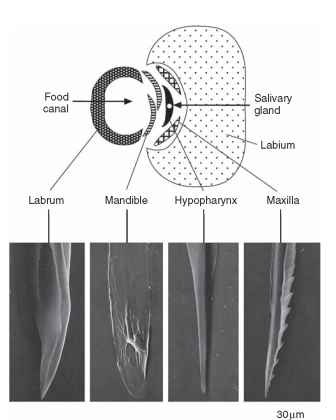
FIGURE 7 Piercing and sucking mouthparts of a female mosquito. Above is a transverse section through the proboscis. Below are electron micrographs of the tips of the stylets.
of the labellum in a series of rasping movements that enable them to tear through the host’s skin. For example, compare Fig. 8B, which depicts the rasps and spines on the inside of the labellar lobe and pointing downward, with Fig. 8C, in which they are on the outside and pointing upward. Contact chemoreceptors are also exposed as the teeth are moved around the tip of the labellar lobes so that they are in a position to detect blood as the host’s capillaries are damaged.
Once the insect starts to feed, the properties of the fluid and dimensions of the food canal affect the rate of uptake and so the rate of nutrient intake. The more viscous a fluid, the more slowly it flows, so that although nectar containing high concentrations of sugars has more nutrients per unit volume, it also is taken up more slowly than a more dilute solution. The flow rate is also negatively correlated with the length of the proboscis, but positively correlated with the diameter of the food canal; the greater the diameter of the canal, the faster the fluid flows.
Movement of fluid into the gut is affected by three factors: the hydrostatic pressure of the fluid in the host organism, capillarity, and muscular activity. If the fluid is under high pressure, simply piercing the vessel containing it is sufficient for the fluid to be forced out, just as water gushes out from a burst water main. Phloem, the fluid carrying sugars and amino acids away from photosynthetic tissues to other parts of a plant, is under such positive pressure, up to 1 MPa, and, consequently, phloem-feeding insects, such as most aphids, have simply to penetrate a sieve tube and the phloem is forced through the food canal and into the gut. If the stylets are cut experimentally, phloem continues to ooze out, and this oozing provides a method for obtaining samples of phloem. Although vertebrate blood is under
![Piercing and sucking mouthparts of a tsetse fly. (A) Longitudinal section through the tip of the labellum at rest. The rasps are internal, and prestomal teeth are concealed within the opening. Arrows indicate the directions of movement of the rasps when the labellar lobes are everted. (B) Inside of one labellar lobe in the rest position. Each rasp is made up of rows of downwardly pointing cutic-ular spines. [A and B reproduced, with permission from Cambridge University Press, from Jobling, B. (1933). A revision of the structure of the head, mouth-part and salivary glands of Glossina palpalis Rob-Desv. Parasitology 24, 449-490.] (C) Side view of the labellum with rasps everted. The rasps and prestomal teeth are now on the outside of the labellar lobe. Notice that the teeth now point upward. In moving from the position shown in (A) and (B), the pointed ends have scraped the skin of the host. This movement, and the anatomical arrangement, are basically similar to that seen in the blowfly (Fig. 6). Piercing and sucking mouthparts of a tsetse fly. (A) Longitudinal section through the tip of the labellum at rest. The rasps are internal, and prestomal teeth are concealed within the opening. Arrows indicate the directions of movement of the rasps when the labellar lobes are everted. (B) Inside of one labellar lobe in the rest position. Each rasp is made up of rows of downwardly pointing cutic-ular spines. [A and B reproduced, with permission from Cambridge University Press, from Jobling, B. (1933). A revision of the structure of the head, mouth-part and salivary glands of Glossina palpalis Rob-Desv. Parasitology 24, 449-490.] (C) Side view of the labellum with rasps everted. The rasps and prestomal teeth are now on the outside of the labellar lobe. Notice that the teeth now point upward. In moving from the position shown in (A) and (B), the pointed ends have scraped the skin of the host. This movement, and the anatomical arrangement, are basically similar to that seen in the blowfly (Fig. 6).](http://lh6.ggpht.com/_X6JnoL0U4BY/S8HbvQhnpAI/AAAAAAAAYVs/nWGNt_u5WRQ/tmp98_thumb_thumb.jpg?imgmax=800)
FIGURE 8 Piercing and sucking mouthparts of a tsetse fly. (A) Longitudinal section through the tip of the labellum at rest. The rasps are internal, and prestomal teeth are concealed within the opening. Arrows indicate the directions of movement of the rasps when the labellar lobes are everted. (B) Inside of one labellar lobe in the rest position. Each rasp is made up of rows of downwardly pointing cutic-ular spines. [A and B reproduced, with permission from Cambridge University Press, from Jobling, B. (1933). A revision of the structure of the head, mouth-part and salivary glands of Glossina palpalis Rob-Desv. Parasitology 24, 449-490.] (C) Side view of the labellum with rasps everted. The rasps and prestomal teeth are now on the outside of the labellar lobe. Notice that the teeth now point upward. In moving from the position shown in (A) and (B), the pointed ends have scraped the skin of the host. This movement, and the anatomical arrangement, are basically similar to that seen in the blowfly (Fig. 6).
pressure, pressure in the blood capillaries is probably too low to play a major part in forcing blood into the insect’s gut.
Xylem, in contrast to phloem, is under high negative pressure which may exceed -1 MPa. [Xylem is the fluid imbibed by the roots of plants and drawn upward through xylem vessels as a result of water loss (transpiration) from the leaves.] Consequently, insects that feed on xylem require a powerful pump to overcome this negative pressure and draw the fluid into the mouth. Cicadas have a highly developed cibarial pump made obvious externally by the inflated clypeus. The cibarium is the space between the mouthparts, outside the mouth. In fluid-feeding insects, this space forms a continuum between the mouthparts and the mouth. Blood-sucking insects such as mosquitoes also have a cibarial pump, but it is less well developed than the pharyngeal pump formed by the first part of the foregut.
The importance of capillarity in insect feeding is not well understood. Insect cuticle, in general, tends to be water repellent but, if the cuticle lining the food canal in the mouthparts is wettable, capillarity might be important. In honey bees, when the glossal tongue is dipped into nectar, the fluid adheres to it, being held in place by hairs that project from the surface of the tongue. The glossa is drawn in and out between the folds of the galeae and labial palps and it is probable that capillarity is important in drawing the fluid toward the mouth.
