Among all terrestrial animals, only vertebrates and insects are L\ richly endowed with a sense of hearing. By “hearing,” we usually mean the ability to detect minute, time-varying changes in air pressure that we familiarly experience as “sound.” Under this restricted definition, we can say that audition has evolved in at least seven orders of insects, including all of the major orders except the Hymenoptera (wasps, ants, bees). However, if we were to include under “hearing” the ability to detect sound waves in water and solids, or the displacement of molecules in a sound’s near field, then the number of “auditive” insects would expand enormously to include not only the Hymenoptera but even small orders such as Plecoptera (stone flies) and Isoptera (termites). Initially, we focus on the form and function of tympanal ears, which are organs that are sensitive to sound signals that are propagated through the air or water as fluctuations in pressure and which come to mind when we (humans) use the term “hearing with ears.” Following this, we provide some examples of nontympanal hearing organs.
Using sound, vertebrates and insects are often capable of sensing, identifying, and locating their predators, prey, conspecific rivals, and mates by hearing their intentional or unintentional acoustic signals. As might be expected, natural selection has shaped the form and function of hearing organs (ears) in insects over evolutionary time. In this respect, the ears of insects show much greater diversity than those of vertebrates, for reasons that will be apparent in our discussion. However, it must be emphasized that despite the scope of morphological diversity among insect ears, there is a morphological “bauplan” (structural design) that underlies their great range in behavioral and physiological function.
MORPHOLOGICAL REQUIREMENTS
There is tremendous morphological diversity of insect ears (Fig. 1). The multitude of different ear designs and locations reflects the unique physical and behavioral challenges faced by each insect. Yet despite their many differences, most ears follow a similar morphological plan. Each typically consists of three identifiable substructures: a tympanal membrane, a tracheal air chamber, and a chordotonal sensory organ.
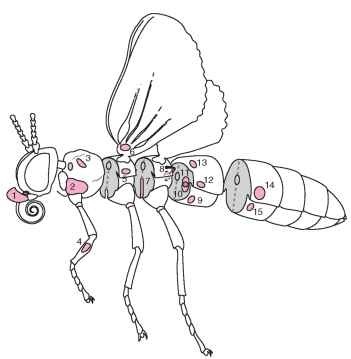
FIGURE 1 A schematic drawing of a generalized insect showing 15 body locations where tympanal ears have been identified. Each number represents a position on the body where an ear has evolved independently in one or more taxa, although all species within a taxonomic division do not necessarily possess ears. (1) Lepidoptera: Sphingidae (Choerocampini, Acherontini); location: palp-pilifer region. (2) Diptera: Sarcophagidae, Tachinidae; location: ventral inflation of prosternum, between coxa. (3) Coleoptera: Scarabaeidae, Dynastinae; location: dorsolateral region of proster-num. (4) Orthoptera: Ensifera: Gryllidae, Tettigoniidae; location: tibia of foreleg. (5) Heteroptera: Hydrocorisae (water boatmen); location: lateral mesothorax, ventral to wing base. (6) Lepidoptera: Papilionoidea, Hedyloidea; Neuroptera: Chrysopidae; location: base of ventral forewing. (7) Dictyoptera: Mantodea; location: within a deep groove between the metathoracic legs. (8) Lepidoptera: Noctuoidea; location: within a cavity on the posterior metathorax. (9) Lepidoptera: Pyraloidea; location: within a cavity on ventral surface of first abdominal segment. (10) Lepidoptera: Geometridae; location: within a cavity on anterior side of first abdominal segment. (11) Lepidoptera: Drepanidae; location: internalized tympanal membrane located between two air-filled chambers on first abdominal segment. (12) Orthoptera: Acrididae; location: lateral surface of first abdominal segment. (13) Coleoptera: Cicindelidae; location: dorsal surface of first abdominal segment, beneath the elytra. (14) Homoptera: Cicadidae; location: within cavity on lateral second abdominal segment. (15) Lepidoptera: Uraniidae; location: within cavity at the anterior (females) or posterior (males) end of the second abdominal segment.
tympanal .Membrane
The tympanal membrane (eardrum) is a thinned region of exoskel-eton, typically supported by a chitinous and stretched across an enlarged air-filled cavity. Sound impinges upon the membrane, setting it and its associated nerve cells into motion. The thickness of the membrane can vary from 1 to over 100 |im. The ultra-sound-sensitive ears of many nocturnal Lepidoptera (butterflies and moths), for example, are so thin that they are transparent. Such fragile membranes are typically protected within body cavities or by external flaps of cuticle. In contrast, the thicker, opaque tympanal membranes of some diurnal butterflies or grasshoppers are conspicuously positioned on the outer surface of the body.
Tracheal Air Chamber
The internal face of the eardrum backs onto an enlarged air-filled chamber, which forms part of the tracheal respiratory system. In some ears, the air chambers are connected directly to other sound input sources (spiracles or contralateral ears) or resonating chambers via the tracheal system. In a few rare cases [e.g., green lacew-ings (Chrysopidae) and some water bugs (Corixidae)] the tympanic chambers are largely fluid filled.
Chordotonal Organ
Associated with the inner surface of the tympanal membrane is one to several chordotonal organs. Chordotonal organs are specialized mechanoreceptors unique to insects and crustaceans, but not unique to ears. Each chordotonal organ comprises one or more individual sensory units called scolopidia, and each scolopidium consists of three cells arranged in a linear array: a sensory cell, a scolopale cell, and an attachment cell (Fig. 2A). The total number of scolo-pidia in an ear ranges from one in some moths (Notodontidae) to almost 2000 in the bladder grasshopper (Bullacris membracioides). A chordotonal organ may attach directly to the inner surface of the tympanic membrane (e.g., Fig. 4E) or to tracheal air sacs indirectly associated with the tympanum. The axons of the sensory neurons collectively make up the auditory nerve that forms the neural link between the mechanosensory stimulation of the eardrum and its “perception” by the central nervous system. Vibrations of the eardrum and/or air chamber cause bioelectric currents to flow in the sensory cell, initiating action potentials in the auditory nerves and signaling neurons in the auditory pathways of the nervous system.
MECHANISMS
Sound waves are fluctuations in pressure traveling through a medium away from a source of mechanical disturbance. Tympanal ears are designed to detect these minute pressure changes traveling through air or water. Depending on the structural design of the ears, they may convey information about the location, frequency, and intensity of an acoustic stimulus.
There are two distinct types of insect tympanal ears: pressure receivers and pressure difference receivers (or pressure gradient receiver). In a pressure receiver, the tympanal membrane forms one side of an otherwise enclosed air chamber. The membrane vibrates in response to sound waves arriving only to the external surface of the tympanal membrane. In insects whose ears are sensitive to high-frequency sounds (like many bat-detecting moth ears), the sound shadow of the body is sufficient to cause a difference in sound amplitudes arriving to each ear, which provides useful directional cues to the animal. Many insects using lower frequency sounds are faced with the difficulty of being able to localize sounds that arrive almost simultaneously to both ears. They overcome this problem using pressure-difference receivers in which the sound impinges on both the outer and the inner surfaces of the membrane, and the membrane vibrates in response to the difference in pressure between the two sides (e.g., Fig. 3).
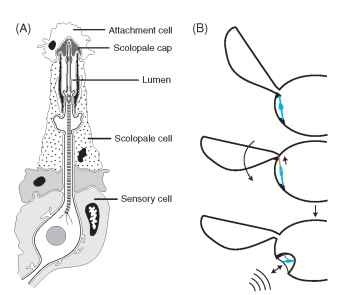
FIGURE 2 Insect tympanal sensory receptors. (A) A typical tym-panal scolopidial organ, consisting of three cell types. The dendrite of a bipolar sensory neuron projects into a fluid-filled space (lumen) formed by the walls of an enveloping scolopale cell. The distal tip of the dendrite inserts into the scolopale cap, an extracellular secretion of the scolopale cell. The attachment cell connects the sensory neuron and scolopale cell to the tympanal membrane, either directly or indirectly via a tracheal air sac. A chordotonal organ may have from one to several thousand scolopidia. (B) A schematic diagram depicting the hypothetical transition from a wing-hinge proprioceptive chordotonal organ to a tympanal hearing chordotonal organ. The top two images show a chordotonal organ functioning as a proprioceptor monitoring wing movements. At the bottom, the chordotonal organ has been mechanically isolated within a rigid tympanal cavity and attaches to a thinned region of cuticle (the tympanic membrane) that detects sounds. (A was modified, with permission, from Gray, E. G. (1960). The fine structure of the insect ear, Philos. Trans. R. Soc. B 243, 75-94.
A third and very unusual way to localize sounds has been “invented” by certain parasitic tachinid and sarcophagid flies. These flies localize their hosts (field crickets, katydids, and cicadas) by hearing and then homing in on the mating calls. The ears possessed by these flies are conventional chordotonal organs, containing 70 or more scolopidia. However, the fly’s eardrums are mechanically connected, which confers an acute sense of directionality such that they can detect and localize their calling hosts/prey from a distance of tens of meters, even from high in the air. Such ears appear to be unique to these parasitoid flies.
The frequencies detectable by insect tympanal ears range from a few kilohertz (e.g., the water boatman Corixa) to over 100kHz (e.g., some tettigoniid species). This broad bandwidth of frequency sensitivity is used by some insects for mate calling and in others it enables them to detect their predators (e.g., echolocating bats). Some insects have the ability to discriminate between different sound frequencies, which may be achieved at the level of the tympanal membrane or the chordotonal organ. To date, most insect ears studied appear to be tone deaf (i.e., different tones are indistinguishable).
LOCATION
Ears can be located just about anywhere on insects (Fig. 1). There is one striking difference between insect ears and vertebrate
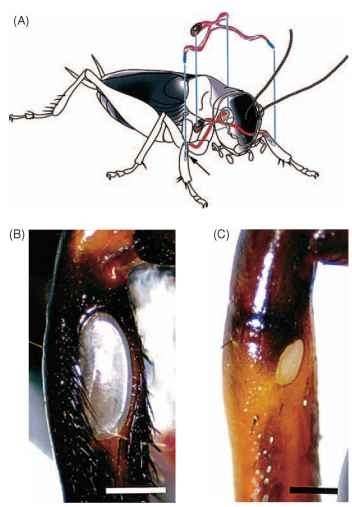
FIGURE 3 Schematic illustrations of structures associated with hearing in crickets (Ensifera). (A) The locations of the tibial ears and the H-shaped tracheal system connecting the contralateral tympanal membranes and spiracles are shown. (B and C) Light micrographs of the larger posterior and smaller anterior tympanal membranes located on the tibia of the forelegs in Gryllus bimaculatus.
ears: those of the latter, because of developmental constraints, are always on opposite sides of the head and always on the head, whereas insect ears have been found on virtually every part of the body. Of course, in any given species of insect, ears are always found on the same part of the body, but from group to group, ears may be found on the head, legs, wings, thorax, or abdomen.
Although the ears of most insects are clearly recognized by the presence of a conspicuous tympanal membrane either on the body’s external surface or within an ear cavity, the ears of other insects are morphologically cryptic. For example, some hawk moths (super-family Sphingoidea) possess hearing organs in their mouthparts, and by inflating their palps while they are feeding on flowers at dusk, they create functional ears that alert them to the echolocation calls of bats. The “cyclopean” ear of the praying mantis is located within a deep groove between the hind legs, and the tympanal membranes are not morphologically distinct from surrounding body parts, even to the trained eye. Even more cryptic are the ears of the Madagascar hissing cockroach, Gromphadorhina portentosa, in which there is no obvious eardrum or tympanal membrane overlying the internal chor-dotonal organs contained within the tibiae of its forelegs.
Although most insects possess a single pair of hearing organs, there are at least two reports of insects with multiple ears. One lineage of praying mantids has two sets of ears: one located between the mesothoracic legs and tuned to ultrasonic frequencies (25-40 kHz) and another between the metathoracic legs and tuned to lower frequencies (2-4kHz). The multieared bladder grasshopper (B. mem-bracioides) of South Africa has six pairs of serially repeated abdominal ears that function in detecting mating calls at distances of ~2km.
DEVELOPMENTAL ORIGINS
In vertebrates, ears are always located on the head, behind the eyes, and above the jaw, but in insects they are virtually anywhere on the body. Why are they not confined to one place like those of vertebrates? Evolutionary and developmental origins of the vertebrate ear, drawing from embryological anlagen (organs or structures in their earliest state) such as the gill arches, neural crest, and the otic capsule, have constrained the ears to their canonical position in the head. Insect ears require two structural modifications, cuticular and spiracular, combined with innervation by a chordotonal organ. In the insect body, chordotonal organs do not function only as hearing organs, but are actually widely distributed throughout the body, where they act as detectors of self-induced body movements (pro-prioceptors) or substrate vibrations. Insect bodies are made up of a series of hard cuticular plates joined by flexible membranes, and chordotonal organs are frequently suspended between moving joints. We now know through developmental studies and comparative anatomy that it is not so difficult to “construct” an ear by making a few peripheral modifications to an existing propriocep-tor and its surrounding cuticular and tracheal structures. By simply thinning the cuticle, enlarging the surrounding tracheal air sacs to allow membrane vibration, and mechanically isolating the sensory organ from body movements, a proprioceptor can be converted into a sound-pressure receiver (Fig. 2B). For example, the bat-detecting ears on the thorax of noctuoid moths are thought to have evolved from proprioceptors monitoring wing movements. Given the jointed, segmental body plan of any insect, the ubiquitous branching of the respiratory tracheae lining the inner face of the cuticle, and the widespread occurrence of chordotonal organs that span different segments in the body and appendages, the precursors of an insect ear can be found virtually at every joint in the body and appendages. There seem to be few developmental constraints in positioning an ear, should an adaptive need arise.
Given so many possibilities for developing an ear, how does it come about that a particular insect possesses an ear in one place, say its forelegs, and another species in another, say its abdomen? One can imagine a host of anatomical, biophysical, ecological, and evolutionary constraints and advantages that might play important roles in the selection process. Such factors as the distances between the ears, the degree of protection offered by surrounding structures, the availability of tracheal air sacs, or the preexisting connections to the central nervous system may all be important. For example, if the function of hearing in a flying nocturnal insect like a moth is to detect and avoid echolocating bats, then a proprioceptor with prees-tablished neural connections to wing flight musculature would be a better ear candidate than, say, a leg proprioceptor. Or, possibly, the reason the thorax and abdomen are so “busy” with ears (Fig. 1) could be because these locations offer maximum interaural distance and a high degree of protection.
EVOLUTION
Tympanal ears have evolved independently at least 20 times within the class Insecta, and this number surely underestimates the number of ears that do exist. Still, hearing appears to be the exception, not the rule, for insects, with only 7 of the 25 recognized extant neopteran orders having tympanate species. The Orthoptera (crickets, grasshoppers, and katydids) and the Lepidoptera (moths and butterflies) boast the largest number of eared species. In two of the most spe-ciose orders, the Diptera (flies and mosquitoes) and the Coleoptera (beetles), tympanal ears are rare, and surprisingly, the Hymenoptera (wasps, ants, and bees) are completely atympanate as far as we know. Following is a brief introduction to the major taxonomic groups for which tympanal ears have been identified to date.
Orthoptera
Orthopteran ears come in two different forms and are divided nicely by the taxonomy of the order. The Acridoidea (locusts, grasshoppers) have ears on either side of the first abdominal segment. The tympanal membranes are nearly circular, opaque, and clearly visible upon inspection with the naked eye. Tracheal sacs connect both ears, allowing the locust to determine the direction of a sound source. The acridid ear is one of the few insect ears known to have the capability of pitch discrimination. About 60-80 scolopidia form four separate groups that attach to different regions of the eardrum, which, in turn, resonate to different sound frequencies. The ears of extant acridids function primarily in conspecific communication, but comparative evidence suggests that the primitive function was for predator detection.
“n the suborder Ensifera [crickets (Gryllidae), katydids (Tettig-oniidae)] the ears occur just below the “knee” region, on the tibia of the forelegs. Each leg has two eardrums—one on each side of the leg (Fig. 3). The tympanal membranes are connected to other sound input sources (the spiracles, contralateral ear) via a system of tracheal tubes and air chambers, which play important roles in directional hearing. The ensiferan auditory chordotonal organ (crista acoustica) has typically between 60 and 80 sense cells arranged in a linear array down the leg, connecting indirectly to the tympanal membrane by tracheal air sacs. Like the acridid ear, the ensiferan ear is capable of pitch discrimination. Presumably, the function of hearing in primitive Ensifera was conspecific communication, which remains the primary function in extant species. Some species are sensitive to ultrasound and use their ears to detect bats in addition to communicating with conspecifics. Fossil records demonstrate that the ancestors of modern Ensifera, which predate the appearance of bats by at least 100 million years, had ears on their legs and therefore, ultrasonic hearing for defense against bats seems to have evolved secondarily.
Lepidoptera
Tympanal ears have evolved more times within the Lepidoptera than any other insect order. To date, at least seven ears of independent origins have been described. Ears are located in the mouthparts (Sphingoidea), at the base of the forewings (butterflies: Hedyloidea, Nymphalidae), in the thorax (Noctuoidea), or on anterior abdominal segments (Tineoidea, Pyraloidea, Drepanoidea, Geometroidea, Uraniidae). Moth ears are among the simplest of all insect ears in that they have very few auditory sensory cells (one, two, or four). K. D. Roeder first demonstrated that the primary function of hearing in moths is to detect the ultrasonic echolocation cries of insectivorous bats. Despite their simplicity, moth ears are capable of determining the distance and direction of an approaching bat. Flying moths evade bats by either turning away from a distant bat or engaging in evasive and erratic flight maneuvers to avoid a sudden and unexpected attack. W. E. Conner has shown that some species, including many day-flying tiger moths (Arctiidae), use their hearing in social interactions. In most Arctiidae, ultrasound production functions to either jam a bat’s echolo-cation calls or warn the bat of a distasteful meal (aposomatic display), but in those species that use sounds for social interactions, both hearing and sound production have secondarily taken on different roles.
Hearing in butterflies has only begun to be explored. The hedylid butterflies of the neotropics, unusual because of their nocturnal habits, have ultra-sound-sensitive ears on their wings that function as bat detectors (Fig. 4). Some diurnal butterflies of the family Nymphalidae possess earlike structures on their wings, and there is evidence that the “cracker” butterfly (Hamadryasferonia) uses its ears for conspecific communication. No doubt there will be further examples of butterfly hearing in the near future, since tympanal-like structures have been described anatomically in many species.
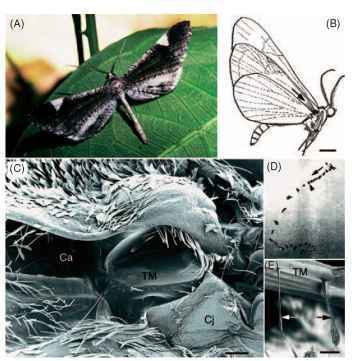
FIGURE 4 Nocturnal butterflies of the superfarmly Hedyloidea possess ultra-sound-sensitive ears on their wings that mediate evasive flight maneuvers to avoid bats. (A) A male Macrosoma heliconiaria (Hedylidae). (B) Lateral view of M. heliconiaria, showing the general location of the right ear. An arrow points down the canal toward the tympanic cavity where the tympanic membrane resides. Scale bar, 3 mm. (C) Scanning electron micrograph of the right tympanal ear. The hind wing and a dense fringe of scales have been removed to reveal the tympanic cavity. Ca, canal; Cj, conjuctiva. Scale bar, 110 |im. (D) Consecutive video images (30 frames/s) of a free-flying M. heliconiaria responding to a short (~250ms), high-frequency (25kHz), high-intensity (>100dB) sound. The direction of flight is marked with an arrow and the stimulus onset with an arrowhead. (E) Scanning electron micrograph of the two most proximal chordotonal organs (arrows), viewed from inside the tympanic chamber. The largest organ attaches to the proximal border of the tympanal frame (black arrow). TM, tympanic membrane. Scale bar, 50 |im. (A and E courtesy of J. Yack. B-D were modified, with permission, from Yack. J. E., and Fullard, J. H. (2000). Ultrasonic hearing in nocturnal butterflies. Nature 403, 265-266.)
Homoptera and Heteroptera
Most people are familiar with the loud “buzzing” sounds of cicadas (Cicadidae) during hot weather; these are typically males calling to females. Cicada ears are located within cavities on the ventral side of the second abdominal segment. They are among the largest of all insect ears, with over 1000 scolopidia in each ear. It has been suggested that the large number of sense cells enhances the ear’s sensitivity for long distance communication, but cicadas do not appear to have more sensitive hearing organs than other insects with far fewer scolopidia.
Water bugs (suborder Hydrocorisae) are reported to have ears on various body parts, including the mesothorax, metathorax, or first abdominal segment. The best known is the ear of the water boatman, Corixa (Corixidae), which occurs on the lateral mesothorax between the wing and the leg. The ears, like those of moths, are simple, with only two auditory cells, both tuned to low-frequency sounds (1-2 kHz), within the range of conspecific calls. The insect carries a bubble of water with it to allow the membrane to vibrate under water. Unlike for most other insect ears, the tympanal membrane is backed by fluid, not air. Hearing in corixids appears to function primarily for mate attraction.
Diptera
Although it had been well documented that certain parasitic flies were attracted to the songs of crickets, katydids, and cicadas, until recently, it was not known how these flies were eavesdropping on their hosts. Two families of parasitoid flies (Sarcophagidae and Tachinidae) have independently evolved a pair of peculiar ears on their prosternum, just below the head, in the “neck” region (Fig. 5). The gravid females use their ears to locate singing insect hosts upon which to lay their predaceous larvae. The ears of the two para-sitoid groups are described earlier in this article. The design features of these dipteran ears show remarkable convergence in anatomy and function, despite the fact that tachinids and sarcophagids are only distantly related. This suggests that evolutionary and developmental constraints are at work here in ways that we do not yet understand.
Until recently, praying mantids were thought to be deaf; now we know that 65% of all mantid species can hear. The ears occur in the most bizarre location: the two tympanal membranes face one another inside a narrow groove between the metathoracic legs. The mantid ear is functionally an “auditory cyclops” because the close proximity of the eardrums (less than 150 |im) provides no directional
information to the animal. The ears function as bat detectors and are most sensitive between 25 and 50 kHz.
Coleoptera
Tympanal ears have been described in two beetle families to date. Several species of the genus Cicindela (Cicindelidae) have ears on the dorsal surface of the first abdominal segment, beneath the wings. Some scarabs (two tribes in the subfamily Dynastinae) have ears located just beneath the neck membranes (pronotal shield). The ears of both families are tuned to ultrasonic frequencies, and there is strong evidence that they function as bat detectors. It is a little bit surprising that there are not much examples of hearing in beetles. Given the large number of species, the wide diversity of niches worldwide, and numerous reports of sound production, we expect that more examples will be uncovered.
Neuroptera
Green lacewings (Chrysopidae) have an ear near the base of each forewing in a location similar to that of the ears of some butterflies. The ear consists of a swelling of the radial vein, with a region of very thin cuticle on the ventral side that functions as a tympanal membrane. Like the corixid ear, the tympanal chamber is predominantly fluid filled. The ears respond to sounds between 40 and 60 kHz and are sufficiently sensitive to detect echolocating bats at close distances.
NONTYMPANAL HEARING ORGANS
Until now we have focused on tympanal ears, which are sensitive to traveling waves of changing pressure in air and water, known as the acoustic far field. In the broadest sense of the word, however, hearing encompasses the detection of near-field sounds, as well as vibrations traveling through solid substrates. By and large, the near field can be thought of as a short distance, a few body lengths, from the sound source. Substrate vibrational signals have also been described as “seismic communication.” In this larger sense, then, we could argue that most insects can hear. We highlight a few recent developments in the study of these alternative, but no doubt widespread, forms of hearing.
Detecting Near-Field Sounds
When a sound is produced, air particles are being pushed back and forth near the source of disturbance. These particle movements, or near-field sounds, are generally of low frequency (typically below
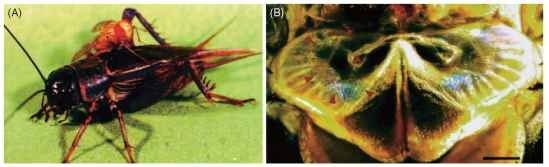
FIGURE 5 The prothoracic tympanal ear of a parasitoid tachinid fly, Ormia ochracea (Tachinidae). (A) A female fly resting on its host (Gryllus integer); (B) a light micrograph of the prosternal ear in a female. The outline of the tympanal membrane is indicated with arrows.
1 kHz) and, unlike pressure waves, do not travel far from the sound source, in many instances, just a few body lengths in distance. A small, light, and ponderable object occurring within the near-field sound will move in response to the vibrating air molecules. In insects, loosely attached setae or antennae are commonly used for detecting particle velocity. Depending on the structure of the receptor organ, and its position relative to the sound source, near-field receptors can offer information about the direction and the intensity of a sound source.
Some caterpillars can detect the near-field sounds produced by the beating wings of a flying wasp up to distances of 70 cm. Specialized hair on the dorsal thorax of the caterpillar are displaced in the sound’s near field, eliciting an evasive response, such as freezing or dropping from a leaf. Similar particle-displacement-sensitive setae on the cerci of crickets and cockroaches function in predator avoidance and possibly for close-range conspecific communication.
The antennae of many insects also function as near-field sound detectors. In many Diptera (mosquitoes, chironomids, and fruit flies), for example, the males are attracted to the near-field “buzzing sounds” of females (Fig. 6). The long flagella of the antennae in male mosquitoes resonate to the tune of flying females, and these antennal movements in turn stimulate many thousands of scolopidia in the Johnston’s organ, a chordotonal organ located at the base of the antennae. The Johnston’s organ may also be involved in the detection of near-field sounds produced by honey bee waggle dances. It is also the sensory organ by which the well-known Drosophila melanogaster detects the patterned wingbeats that make up the mating songs of these species.
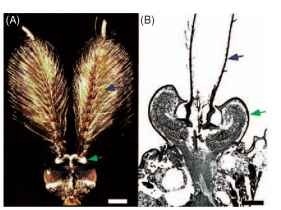
FIGURE 6 The near-field sound hearing organs of a mosquito. (A) Micrograph of the head of a male Toxorhynchites brevipalpis, showing the plumous flagella of the antennae (blue arrows), which resonate in response to the near-field sounds of flying females. (B) A cross section through the antennal base, showing the location of the Johnston’s organ (green arrows), where the auditory scolopidia are located. (Part A is courtesy of D. Huber. Part B was adapted, with permission, from Gopfert, M. C., and Robert, D. (2001). Active auditory mechanics in mosquitoes, Proc. R. Soc. London B 268, 333-339.)
Detecting Substrate-Borne Vibrations
The detection of vibrations traveling through solid substrates may be one of the most ubiquitous but least appreciated forms of acoustic communication in insects. With recent technological advances in the detection of substrate-borne signals (e.g., laser vibrometry and piezoelectric sensors), we are learning that a large number of insects can detect vibrations produced by both intentional and unintentional senders. In fact, most insect orders probably include species capable of detecting vibrations. At present, however, there are few complete studies on this subject. We provide two recent examples out of many possibilities.
Membracid treehoppers (Homoptera: Membracidae) transmit alarm calls through tree stems to communicate with conspecifics. Nymphs living in colonies of up to 100 individuals on the stem of a host plant produce coordinated waves of vibrations when threatened by a predator. The mother treehopper detects these alarm calls and rushes to defend her offspring, by kicking her hind legs and fanning her wings at the intruder (Fig. 7A). For many species of caterpillars that live in social or crowded conditions, vibrational signaling may be the principal means of communication. The common North American masked birch caterpillar (Drepana arcuata) engages in acoustic “battles” with invading conspecifics. The nest owner drums and scrapes its mandibles and scrapes modified “oars” against the leaf in ritualized acoustic displays. Acoustic “duels” between residents and intruders can last from a few minutes to a few hours (Fig. 7B). At present, we know little about how insects detect substrate vibrations. The subgenual organ (a chordotonal just “below the knee” in many insects) functions as a vibration receptor in some groups (like some crickets and termites), but for most insects, the receptor organs are yet to be identified. Clearly further research is required before we gain a full appreciation of this important form of communication in insects.
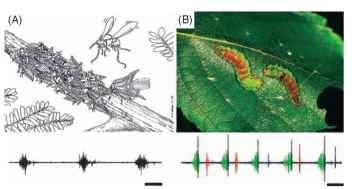
FIGURE 7 Acoustic communication through substrate-borne vibrations. (A) A group of treehopper nymphs (Umbonia crassicornis) use coordinated substrate-borne vibrations to warn their mother of an approaching predatory wasp. The mother detects the signals and rushes to the defense of her offspring. The waveform represents three group signals from an aggregation of nymphs. Scale bar, 560 ms. (Reproduced with permission from Cocroft, R. B. (2002). Antipredator defense as a limited resource: Unequal predation risk in broods of an insect with maternal care, Behav. Ecol. 13(1), 125-133. Waveform courtesy of R. B. Cocroft.) (B) Two masked birch caterpillars (D. arcuata) engaged in an acoustic “duel” over a silken nest on a birch leaf. The waveform depicts the three signal types (green, anal scrapes; blue, mandible drumming; orange, mandible scraping) used by the caterpillars.
FUNCTION
Because of their physical properties, acoustic signals are highly adaptive for certain kinds of behavioral interactions: sound waves can travel at any time of the day or night, through thick vegetation or muddy water; they convey information instantaneously and can be transmitted over long distances; and sounds are easy to localize, do not leave lingering traces, and can transmit large amounts of information per unit time. For the majority of insects, acoustic communication functions primarily in reproductive behavior and predator avoidance, but may also be used for detecting prey or host species (parasitic flies, wasps; predatory water striders, ant lions) or calling to conspecifics to form aggregations (sawfly larvae) or warn of danger (termites, treehoppers).
For humans, the most conspicuous sounds commonly heard from insects are the loud chirps and trills of field crickets, the long raspy choruses of katydids by night, and the intense, shrill-like buzzes and rattles of cicadas by day. These are the mating calls emitted by males in order to attract conspecific females. Sounds used in reproductive interactions function in species recognition, courting, pair maintenance, female mate choice, and male-male competition. The hearing organs of crickets, katydids, grasshoppers, mosquitoes, and cicadas are used primarily for these purposes and are sharply tuned to the calls of conspecifics. The features of these mating calls have surely been shaped by sexual selection.
Many insects have ears for the sole function of detecting predators. Many nocturnally active insects (most Lepidoptera, some mantids, beetles, and lacewings) have ears tuned to the ultrasonic vocalizations of insectivorous bats that use biosonar to detect and home in on their prey. Unlike ears specifically designed for conspecific communication, the ears of predator detectors are usually more broadly tuned and more simple in their design, sometimes having only a few auditory cells per ear.
CONCLUSION
Since antiquity we have known that many insects produce sounds, but only during the past 150 years have scientists realized that some insects can hear. Detailed descriptions of ear anatomy, and the behaviors associated with hearing, began in the early 1800s, providing the basis for current developments in the field of insect bioacous-tics. Over the past 40 years there have been significant advances in the field: many new ears have been discovered, and previous claims to tympanal hearing (based on morphological studies) have been validated. With the development of new instruments for detecting acoustic signals outside the realm of human perception (e.g., ultrasound, solid-substrate-borne vibrations), and for recording neuro-physiological responses to sound, we are now beginning to better appreciate the immense diversity of insect sound receptor organs.
There is still much to learn about insect hearing. We know little, for example, about the chain of physical and bioelectrical events leading to sound reception at the level of the auditory cells or how acoustic sensory responses are integrated at the level of the central nervous system to promote adaptive behaviors. New tympanal ears will no doubt turn up in the years ahead, but perhaps most significantly, future explorations into substrate-vibrational and near-field sound communication are sure to yield exciting insights into how insects communicate acoustically.

